Polarized reflectance of salty flash-frozen ice particles
- 1University of Bern, Phisics Institut, Space Research and Planetary Sciences, Bern, Switzerland (romain.cerubini@space.unibe.ch)
- 2Université Grenoble Alpes, CNRS, IPAG, 38000 Grenoble France
Introduction: In-situ and telescopic observations of icy moons during the last decades have improved our understanding of these bodies. One of the main features discovered is the production of pristine material at their surfaces by plumes, detected on Enceladus[1, 2] and possibly on Europa [3]. In such dynamical environment, the ice particles could be produced by flash-freezing of aqueous solutions expulsed from the subsurface [4, and references therein].
At the University of Bern, the Setup for Production of Icy Planetary Analogues (SPIPA) [5] allows us to produce flash-frozen particles by pulverization of liquid salty solutions into liquid nitrogen. The freezing of the particles is dominated by the Leidenfrost effect [6] which generates a nitrogen outgassing that makes droplets levitating above it. The first seconds of crystallization are made at low (and rapidly decreasing) temperature, inducing a complex internal structure of the particles (mixed of amorphous and crystalline phases).
The characterization of icy surfaces in total light intensity can be complemented by the analysis of the degree of linear polarization of the light scattered by atmosphereless bodies [7]. For many years, ground-based polarimetric observations have provided a consequent dataset to characterize icy surfaces [8, 9] allowing a better preparation for the upcoming space missions Europa Clipper (NASA) and JUICE (ESA).The polarized reflectance is highly sensitive to the morphology of grains (e.g. size, shape, structure) as much as the chemistry (e.g. composition and mixture) [10]. The evolution of polarimetric properties of salty ice particles during warming-up, accompanied with the transition of the amorphous fraction into crystalline, gives insights on the morphology (mostly internal, but also external) of the grains, for the considered salt. Therefore, we have measured the evolution of the degree of linear polarization (Q/I) of salty ices with temperature raising until the eutectic point of the solutions (NaCl, MgCl2, Na2SO4 and MgSO4).
POLICES setup: The POLarimeter for ICE Samples has been developed at the University of Bern to measure, among others, the degree of linear polarization of icy samples [10] and its dependence to phase angle. The experiment is a goniometer with a fixed emergence angle. The incidence angle is defined by the position of the light source, fixed at the end of a rotating motorized arm. Light is either produced by LEDs at three different wavelengths (530, 625 and 810 nm), or in another configuration with a monochromator to select narrow spectral bandpasses. In both cases, depolarizers are used to ensure an unpolarized incident light.
To permit repeatable measurements of icy samples, a hermetic box, painted in black to mitigate stray light, has been added to the setup in order to control the relative humidity and prevent frost formation (Fig. 1A). As depicted in Fig. 1B, polarimetric phase curves can be measured with a phase angle ranging from 1.5° to 73° inside the box [10].
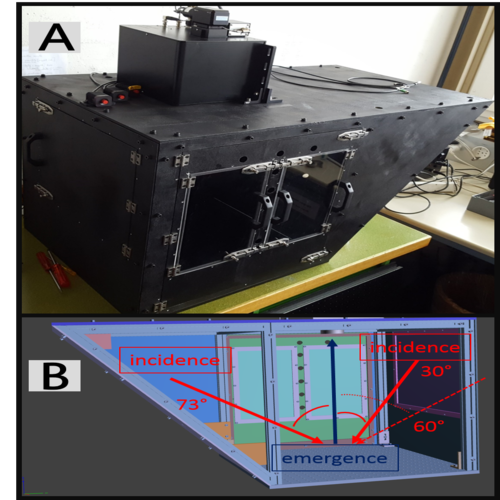
Figure 1: (A) POLarimeter for ICE Samples (POLICES). (B) 3D Back view with geometry of observation
Sample preparation and measurements: The degree of linear polarization is maximum at 90° of phase angle [7]. This configuration is not reachable with our setup; 60° phase angle is a good compromise to record the evolution of linear polarization. We prepared salty ices made of spherical grains 67±31 µm large [5] from saturated aqueous solutions (30wt% of NaCl, 30wt% of MgSO4) and less concentrated solutions, with MgCl2 and Na2SO4.
The ice was placed inside POLICES on a copper plate precooled with liquid nitrogen. The Stokes coefficientswere continuously measured during the slow warming-up of the sample. A Pt-100 sensor inside the ice sample continuously measured the temperature of the ice.
Figure 2 shows the evolution of the Q/I values of the ices with increasing temperature. The evolution observed for a given salt seem to be rather independent on the studied wavelengths so far, with only small offsets of the Q/I values. The pristine material is, as far as understood for now, a mixture of amorphous phase of water and salt, as much as a partially crystallized water ice, in hexagonal and potentially cubic phase [11]. The evolution with temperature corresponds to the reorganization of the sample, leading to a higher degree of crystallinity of both the ice and the salts.
The first steps of growth of these crystals (water ice and hydrated phases of salts) could be responsible for a minute fraction of Rayleigh scattering, associated to particles that are small compared to the wavelength. This mechanism is suspected to be responsible for the peaks of polarization at 175 K for the MgSO4 and centered around 195 K for NaCl (Fig. 2).
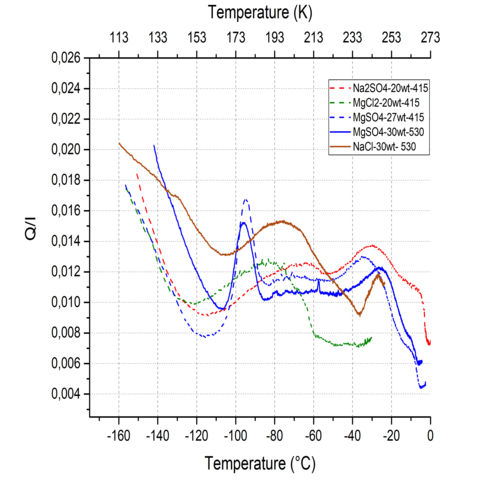
Figure 2 : Evolution of the degree of linear polarisation of salty ices. Here, for clarity, only MgSO4 is shown at 530 and 415 nm.
Summary and perspectives: The new series of measurements proposed in this work will complete the analyses of such brines in total light intensity. We intend to pursue this work with different amount of salts inside the initial brines, as well as other chemical components relevant for icy moons. In addition to measurements at a fixed phase angle, phase curves will be measured. Once validated, polarisation data will be distributed through the DACE and SSHADE platforms (https://dace.unige.ch/lossySearch/; https://www.sshade.eu/db/bypass).
Acknowledgments: The team from the University of Bern is supported by the Swiss National National Science Foundation, in part through the NCCR PlanetS.
References:
[1] Nimmo, F., et al., (2007), Nature 447. [2] Postberg, F., et al., (2009), Nature 459. [3] Roth, L., et al, (2014), Science 343. [4] F. Nimmo, F., et al., (2014), in Encyc. Solar System, Elsevier. [5] Pommerol, A., et al., (2019), Space Sci Rev 215. [6] Feng, H., et al., (2018) International Journal of Heat and Mass Transfer 127. [7] Kolokolova, L., et al., (2015), Cambridge University Press. [8] Kiselev, N., et al., (2009), Journal of Quantitative Spectroscopy and Radiative Transfer, 110. [9] Rosenbush, V., et al., (2015), Polarimetry of Stars and Planetary Systems, 340. [10] Poch, O., et al., (2018), Journal of Geophysical Research: Planets, 123. [11] Uchida, T., et al, (2010), Phys. Chem. Chem. Phys., 12.
How to cite: Cerubini, R., Pommerol, A., Poch, O., Kipfer, K., and Nicolas, T.: Polarized reflectance of salty flash-frozen ice particles, Europlanet Science Congress 2020, online, 21 Sep–9 Oct 2020, EPSC2020-580, https://doi.org/10.5194/epsc2020-580, 2020.

