Equilibration time of Fe & Zn concentrations and isotopes in metal-silicate partitioning experiments
- Vrije Universiteit Amsterdam, Faculty of Science, Earth Sciences, Amsterdam, Netherlands (a.x.seegers@vu.nl)
Introduction
Present geochemical models of planetary core formation commonly focus on either element abundances or isotope fractionation between mantle and core. Data gathered from these two techniques can be mutually inconsistent, leading to different conclusions concerning core formation processes. An example of this is the Si content of the Earth’s core. Element partitioning data and seismological observations indicate that the Earth’s core only contains minor amounts of Si [1], whereas isotopic data suggests significant amounts of Si may be present [9].
To address this data discrepancy problem, this study combines element partitioning behaviour and isotope fractionation of Fe and Zn through metal-silicate partitioning experiments. Metal-silicate partitioning experiments at high pressure and temperature allow for the distribution of elements and their isotopes between planetary cores (metal) and mantles (silicate) to be studied under controlled conditions [e.g. 6,7, 10]. As during planetary core formation it is assumed that some form of equilibrium is reached over millions of years, the experiments should reflect this as well. Therefore, it is essential to determine the time that is required for every experiment to achieve both elemental and isotopic abundance equilibrium.
Approach
Metal-silicate partitioning experiments are performed under high pressure and temperature conditions using an end-loaded piston-cylinder press. To solely focus on the equilibration times, experiments of the same starting compositions were subjected to peak conditions of 1GPa and 1823K for a time ranging from 3 minutes up to 12 hours. The experiments consisted of a synthetic analogue of the lunar Apollo 15 Green Glass as silicate phase [3], along with a metal phase of Fe-metal doped with Zn.
Element concentrations of metal and silicate phases of every experiment were measured with an electron microprobe (EMPA) for major elements and laser ablation ICP-MS (LA-ICP-MS) for trace elements. To analyse Fe and Zn isotopic fractionation, both Zn and Fe were isolated from the metal- and silicate phases through ion-exchange chromatography. The AG-X8 resin was used for the separation of Fe, and the AG-MP-1 resin for Zn [5]. The isotopes of Fe and Zn were subsequently measured using a multi-collector ICP-MS (MC-ICP-MS) with a double spike technique. The precision of the isotope analyses is determined by measuring isotopic reference materials. The standards IRMM-014 (Fe) and ETH-ZN indicate a precision of ± 0.03‰ (2SD) for Fe isotopes and ± 0.05‰ (2SD) for Zn isotopes respectively. Additionally, rock standards such as BHVO-2, BCR-2, AGV-2 and BIR-1 that had been processed exactly like the experiments were measured during the analyses.
Results
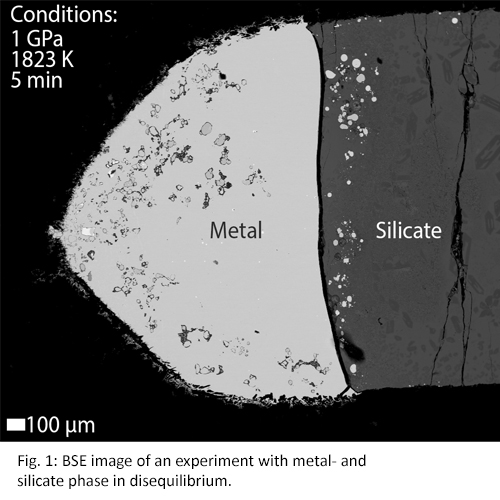
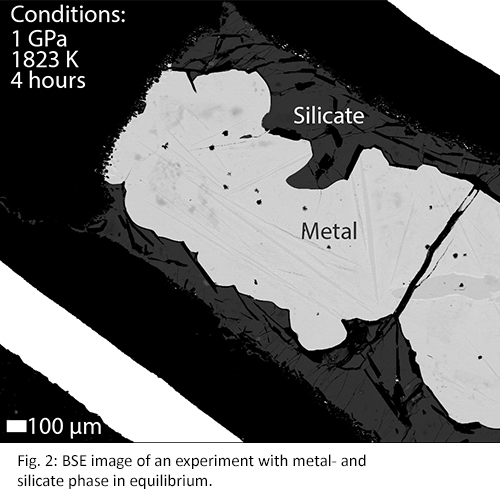
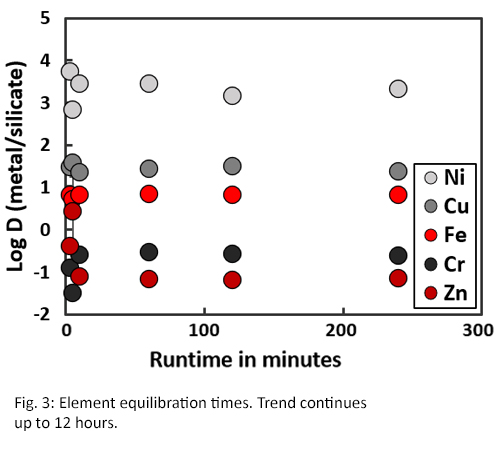
Experimental run products typically show a good separation of a metallic phase and quenched silicate melt. Disequilibrium within experiments can cause heterogeneous spots where groups of elements are clustered. These heterogeneous spots can be seen in experiments of run times of e.g. 3 and 5 minutes (Fig. 1), whereas they are absent in experiments with longer run times (Fig. 2). Results shown in Fig. 3 confirm these observations as the measured elements from short (<10 minutes) experiments show different partitioning behaviour compared to longer experiments (>30 minutes). Therefore, concentration analyses indicate that elemental equilibrium is reached within 30 minutes.
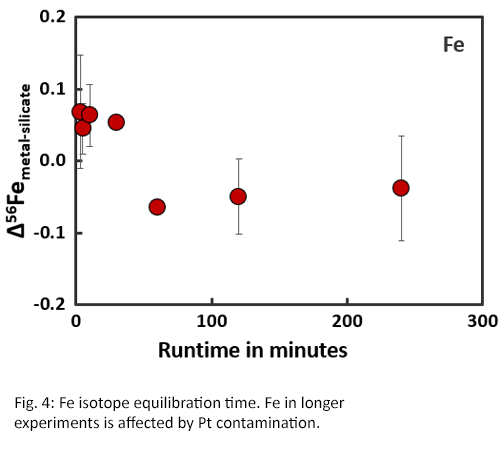
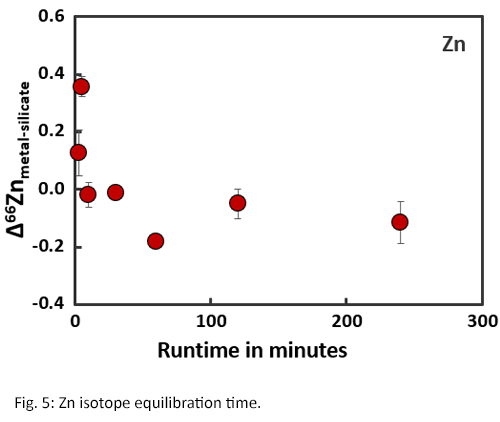
Isotope equilibration times are significantly longer than concentration equilibration times (Fig. 4 and 5) [8]. Within the first two hours of an experiment, the fractionation factors Δ56Femetal-silicate and Δ66Znmetal-silicate are positive and variable. Between 2-4 hours, the fractionation factors become negative and start to stabilise. After approximately 4 hours, isotopic equilibrium is reached for both Fe and Zn with Δ56Femetal-silicate = -0.04‰ ± 0.07 and Δ66Znmetal-silicate = -0.11‰ ± 0.06, which corresponds with literature data [2,4].
Discussion and outlook
Although the data described here cannot be directly applied to planetary core formation models yet, it is essential for creating models of core formation based on a combination of elemental abundances and stable isotope fractionation. This initial data set suggests that there is a relatively uniform Fe and Zn distribution between core (metal) and mantle (silicate) during core formation at high temperature. However, quantification of the effects of pressure, temperature and composition will be needed to further test this hypothesis.
Acknowledgements
We would like to thank the Netherlands Space Office for financial support through its User Support Programme.
References
[1] Badro, J., Côté, AS., and Brodholt, JP.: A seismologically consistent compositional model of Earth’s core, Proceedings of the National Academy of Sciences, 11, pp. 7542-7545, 2014.
[2] Bridgestock LJ., Williams H., et al.: Unlocking the zinc isotope systematics of iron meteorites, Earth and Planetary Science Letters, 400, pp. 153-164, 2014.
[3] Delano, JW.: Pristine lunar glasses : criteria, data and implications, Journal of Geophysical Research, 91, pp. 201-213, 1986.
[4] Hin RC., Schmidt MW., Bourdon B.: Experimental evidence for the absence of iron isotope fractionation between metal and silicate liquids at 1GPa and 1250-1300°C and its cosmochemical consequences, Geochimica et Cosmochimica Acta, 93, pp. 164-181, 2012.
[5] Moeller K., Schoenberg R., et al.: Calibration of the New Certified Reference Materials ERM-AE633 and ERM-AE647 for Copper and IRMM-3702 for Zinc Isotope Amount Ratio Determinations, Geostandards and Geoanalytical Research, 36, pp. 177-199, 2012.
[6] Righter, K .: Metal-silicate partitioning of siderophile elements and core formation in the early Earth, Annual Reviews of Earth and Planetary Sciences, 31, pp 135-174, 2002.
[7] Rose-Weston, L., Brenan, JM., Fei, Y., et al .: Effect of pressure, temperature and oxygen fugacity on metal-silicate partitioning of Te, Se and S: Implications for Earth differentiation, Geochimica et Cosmochimica Acta, 73, pp.4598-4615, 2009.
[8] Roskosz, M., Luais, B., Watson, HC., et al .: Experimental quantification of the fractionation of Fe isotopes during metal segregation from a silicate melt, Earth and Planetary Science Letters, 248, pp. 851-867, 2006.
[9] Shahar, A., Ziegler, K., Young, ED., et al .: Experimentally determined Si isotope fractionation between silicate and Fe metal and implications for Earth’s core formation, Earth and Planetary Science Letters, 288, pp. 228-234, 2009.
[10] Siebert, J., Corgne, A., Ryerson, FJ .: Systematics of metal-silicate partitioning for many siderophile elements applied to Earth’s core formation, Geochimica et Cosmochimica Acta, 75, pp. 1451-1489, 2011.
How to cite: Seegers, A., van Zuilen, K., Stelwagen, R., van Westrenen, W., and Vroon, P.: Equilibration time of Fe & Zn concentrations and isotopes in metal-silicate partitioning experiments, Europlanet Science Congress 2020, online, 21 September–9 Oct 2020, EPSC2020-824, https://doi.org/10.5194/epsc2020-824, 2020

