Dehydration, melting, and recrystallization of calcium sulfates (gypsum, bassanite, anhydrite) in laser-irradiation experiments
- 1Museum für Naturkunde Berlin, Berlin, Germany (christopher.hamann@mfn.berlin; lutz.hecht@mfn.berlin)
- 2Freie Universität Berlin, Institut für Geologische Wissenschaften, Berlin, Germany
Introduction
Understanding the shock behavior of the calcium sulfates gypsum (CaSO4·2H2O), bassanite (CaSO4·0.5H2O), and anhydrite (CaSO4) is essential for understanding hypervelocity impacts into evaporite sediments that are widespread on Earth and which also occur on Mars [1,2]. Most interest focuses on quantification of the impact-induced release of volatiles (H2O and SO2/SO3) to assess its role in the generation or modification of planetary atmospheres [3–11]. Estimates of the amount of gas released from volatile-bearing materials in hypervelocity impact scenarios rely on assignment of the shock-pressure thresholds for incipient and complete vaporization [10]. However, these thresholds are poorly constrained for calcium sulfates, and many studies produced inconsistent to even contrasting results (cf. [5,7,9]). In addition, reports of sulfate impact melts [6] suggest that the shock behavior of the calcium sulfates is more complex than traditionally thought and possibly involves interaction with coexisting silicate impact melts.
Based on our previous study on the interaction between carbonates and silicates in laser-generated melts [12], we report here on the fate of gypsum and anhydrite in laser-irradiation experiments. Specifically, we laser-irradiated gypsum, gypsum–granite, and anhydrite–granite targets to investigate dehydration, decomposition, and melting of the calcium sulfates as well as interaction between calcium sulfates and silicates in an impact-related context.
Materials and methods
We used a continuous-wave fiber laser of 1.07 µm wavelength at Fraunhofer Institut für Kurzzeitdynamik, Freiburg, Germany, to irradiate a gypsum plate (experiments L1130 and L1131) and pulverized gypsum–granite (experiment L1133) and anhydrite–granite (experiment L1132) mixtures in air at 1 bar and room temperature (Fig. 1). The laser emitted a power of 2 or 5 kW for 2 or 5 s, and 1/e2 beam diameters of 6 or 17 mm were used. The laser intensity varied between 8.8 × 102 and 1.8 × 104 W/cm2 and the laser–matter interaction zone was observed using the setup described in [13]. Temperature measurements (Fig. 2) show that peak temperatures were in the range of 1200 K for the gypsum plate and 2200 K for the composite targets. Textural and compositional characterization of the recovered samples employed transmitted-light microscopy, Raman spectroscopy, and scanning electron microscopy.
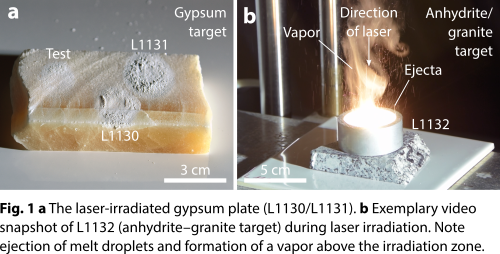
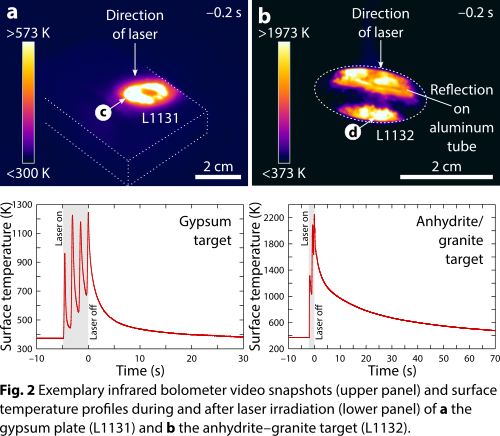
Results and discussion
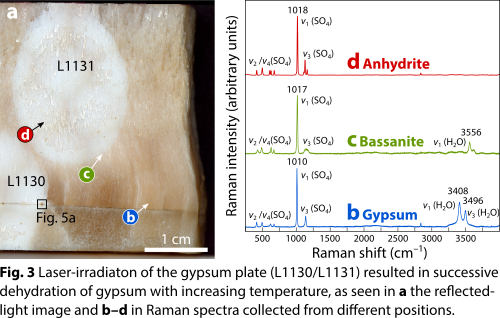
Laser irradiation of the gypsum plate resulted in dehydration of gypsum and successive transformation of gypsum to anhydrite via bassanite. The transformation is evident from the 3250 to 3750 cm–1 region in Raman spectra (Fig. 3), in which the Raman bands corresponding to the stretch vibration modes of water are positioned. Consistent with previous investigations [8,14], the gypsum to anhydrite transformation is also accompanied by a slight upshift of the Raman band associated with the ν1 symmetric stretch vibration modes of the SO4 tetrahedra (i.e., from 1010 cm–1 in gypsum to 1017 cm–1 in bassanite to 1018 cm–1 in anhydrite).
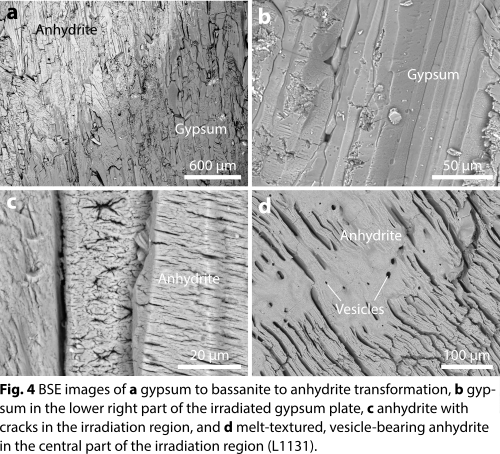
In addition, dehydration of gypsum is also evident from BSE images obtained from the irradiation zones by an increase in brightness (Fig. 4a) and by the presence of cracks in individual anhydrite crystals that are absent in the gypsum crystals of the starting material (Figs. 4b,c). In addition, textures indicative of melting (occurrence of round vesicles; filling of cleavage space by flow-textured material of anhydrite composition) are present in the interior parts of the laser-irradiated regions (Fig. 4d).
Textures indicative of melting and recrystallization of anhydrite were also detected in clast-bearing melt droplets recovered from the gypsum–granite and anhydrite–granite experiments (Fig. 5). Specifically, anhydrite intimately associated with silicate melts occurs in the form of clusters of lath-shaped, typically vesicle-bearing crystallites that often enclose small silicate melt droplets. These clusters are interpreted as quenched CaSO4 liquids. The interfaces between the silicate melts and the anhydrite clusters are typically sharp and reminiscent of menisci formed by liquid immiscibility (cf. [8]; Fig. 5).
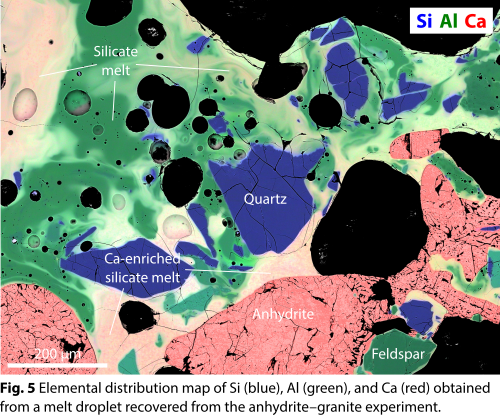
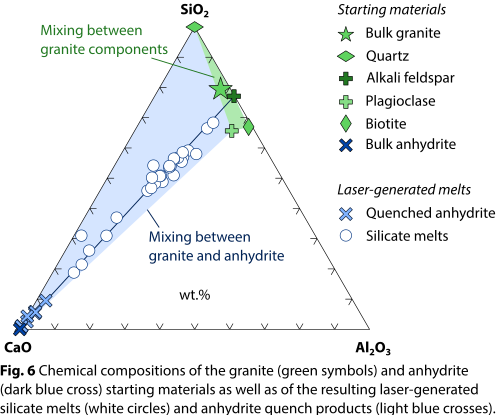
Furthermore, chemical interaction between CaSO4 and the silicate melts derived from the granite is evident from elemental distribution maps (Fig. 5) and point analyses (Fig. 6). Specifically, most silicate melts are heavily enriched in CaO (Fig. 6) as well as SO3, showing data trends in binary (e.g., CaO–SiO2) or ternary (e.g., CaO–SiO2–Al2O3) composition space that cannot be explained by melting and mixing of the granite components alone. In keeping with previous findings [6], limited amounts of SiO2 were detected in the quench anhydrite, suggesting that the CaSO4 liquids and silicate liquids readily exchanged chemical components during the brief time scales (seconds; cf. Fig. 2b) in which the materials were in the liquid state.
Conclusions
Our results suggest that the thermally triggered dehydration of gypsum due to hypervelocity impacts or magmatic activities can be exceptionally fast (seconds to tens of seconds) and results in dehydration of gypsum, formation of anhydrite via basanite, and melting of anhydrite. The presented Raman spectra and microtextures may be useful for interpretation of similar reaction products observed on Mars [2,15–17]. Our results confirm previous observations on shock-melted sulfates [6] and suggest that impact melting of calcium sulfates should be indeed more prevalent than previously thought. Specifically, our experiments illustrate that calcium sulfate impact melts will readily interact with coexisting silicate impact melts, resulting in Ca-enriched silicate melts and Si-enriched, quenched anhydrite melts.
References
[1] Langevin et al. 2005, Science 307, 1584–1586. [2] Vanimann et al. 2018, Am. Mineral. 103, 1011–1020. [3] Chen et al. 1994, Earth Planet. Sci. Lett. 128, 615–628. [4] Pierazzo et al. 1998, J. Geophys. Res. 103, 28607–28625. [5] Gupta et al. 2001, Earth Planet. Sci. Lett. 188, 399–412. [6] Osinski and Spray 2003, Earth Planet. Sci. Lett. 215, 357–370. [7] Skála et al. 2005, GSA Special Paper 384, 413–425. [8] Bell and Zolensky 2011, 42nd LPSC, Abstr. #2008. [9] Prescher et al. 2011, Meteorit. Planet. Sci. 46, 1619–1629. [10] Artemieva et al. 2017, Geophys. Res. Lett. 44, 10180–10188. [11] Kurosawa et al. 2019, Geophys. Res. Lett. 46, 7258–7267. [12] Hamann et al. 2018, Meteorit. Planet. Sci. 53, 1644–1686. [13] Hamann et al. 2016, Geophys. Res. Lett. 43, 10602–10610. [14] Liu et al. 2009, 40th LPSC, Abstr. #2128. [15] Knauth et al. 2005, Nature 438, 1123–1128. [16] Squyres et al. 2012, Science 336, 570–576. [17] Robertson and Bish 2013, Icarus 223, 407–417.
How to cite: Hamann, C. and Hecht, L.: Dehydration, melting, and recrystallization of calcium sulfates (gypsum, bassanite, anhydrite) in laser-irradiation experiments, Europlanet Science Congress 2020, online, 21 September–9 Oct 2020, EPSC2020-869, https://doi.org/10.5194/epsc2020-869, 2020

