Tracking the structural changes of poorly crystallized aluminosilicates by CheMin: A way to analyze the presence of organics in sediments of Gale Crater (Mars)
- 1Departamento de Geociencias, Universidad de Vigo, 36210 Vigo, Spain (duport@uvigo.es)
- 2Centro de Astrobiología (CAB), CSIC-INTA, Carretera de Ajalvir km 4, 28850 Torrejón de Ardoz, Madrid, Spain
- 3Instituto de Investigaciones Marinas, (CSIC). 36208 Vigo, Spain
The formation of methane in Gale Crater remains an intriguing problem due to its sporadic detection and the need to understand its formation and degradation mechanism. Its origin could be biological or inorganic, associated with catalysis facilitated by mineral formations such as serpentines. However, the search for its degradation products, which could remain metastable in the sediments, such as oxalate or acetate, has yet to yield precise results.
The Viking Lander's thermal analyses showed that, although the pyrolysis products generated CO₂ in quantities and intervals compatible with the degradation of organic matter, oxalates should contribute CO2 and CO. At the same time, acetates would likely evolve a mixture of CO2, acetone, and acetic acid. In the case of the CO2 detections by the twin Viking Landers, a lack of indigenous acetone and acetic acid led to rule out the presence of acetates [1].
Subsequent studies [2] discovered that mixing with perchlorate may scrub the acetone and acetic acid evolved by Martian acetates. They demonstrated that this step is not strictly necessary, as thermal decomposition in the presence of oxidants such as perchlorate or peroxide can follow a pattern that generates methane or chloromethane, so the organic origin of CO₂ could not be completely ruled out. The detection limits for acetone were also extremely high for some of the Viking experiments [3]. In addition, chloromethanes were present in Viking data, and the interactions between perchlorates and acetates during heating shifted the perchlorate O2 peaks to lower temperatures, which resulted in better fits with many SAM O2 releases. However, pyrolysis studies are not conclusive.
In addition to SAM, MSL's analytical capabilities include X-ray diffraction (XRD) via the Chemistry and Mineralogy (CheMin) instrument, enabling the identification and quantification of minerals. Another possibility is the determination of these organic compounds as crystalline solid phases using the CheMin diffractometer, which requires intermediate species such as oxalate or acetate to be present in amounts above the 1-2 wt—% detection limit of this instrument [2]. However, so far, the presence of these salts based on the direct determination of their lattice spacings has yet to be observed.
One aspect worth considering is the possibility of methane or its decomposition products, mainly acetate, to be intercalated in the amorphous component or poorly crystalline phases, such as imogolite or halloysite, obtained by recrystallization of aluminosilicates. Exploring this hypothesis could open new avenues for better understanding the formation and persistence of methane on Mars, considering that these compounds could be trapped in amorphous or poorly crystallized mineral matrices, making their direct detection by current methods difficult. Additionally, the possible intercalation of small amounts of these salts in amorphous silicates makes detection via XRD based on the characteristic peaks of the pure crystalline phase very unlikely. However, it opens another detection possibility: the intercalation of compounds such as acetates in these mineral phases produces characteristic and irreversible modifications of the diffraction peaks in the inorganic phase where the intercalation occurs.
To study this possibility, we analyzed the formation of microscopic precursor layers of clay minerals from the nanostructural scale using HREM and SAED as an analog of the formation of secondary minerals through silicate weathering under anoxic conditions. As laboratory analogs of amorphous silicates, we used microtubular structures known as silica-garden that allow the rapid mixing of Si-Al-Fe-Mg-K. Previous experiments with hybrid hydroxide-silica gels showed that the mixing process is one of the determining factors leading to the formation of amorphous silicate multi-oxides (i.e., allophane, imogolite, halloysite). Molecular mixing facilitates further evolution toward more ordered clays. We analyzed the formation of microscopic precursor layers of clay minerals from the nanostructural scale using HREM and SAED.
This process provides the intercalation of hydroxide layers and silica. Furthermore, the formation of the silica garden implies the existence of an extreme change in pH between the internal and external walls of the microtubules, affecting the rate of oxidation of Fe+2 as well as the simultaneous polymerization of silica, providing a way to analyze the influence of the stoichiometry between Si-Fe, the pH, and the rate of oxidation on the formation of clay minerals. Results from these experiments show the presence of curved nanostructures with spacings of 7 Å and 10 Å characteristic of tubular aluminosilicates, such as halloysite, with different degrees of hydration.
In a second series of experiments, dehydrated halloysite (7 Å) was mixed with potassium acetate to produce solid-state intercalation by mixing salt and clay. This experiment produced the appearance of characteristic and irreversible peaks in heating-cooling cycles at spacing values of 9.2 Å and 9.6 Å, depending on the degree of mixing in the intercalation process. This procedure suggests that detailed analysis, using quantitative techniques such as the Rietveld method of poorly crystalline silicates, modified characteristically with organic species like acetate, can be an advantageous method for identifying possible degradation products of organic phases, compared to searching for them as independent solid phases, due to their low detection limit.
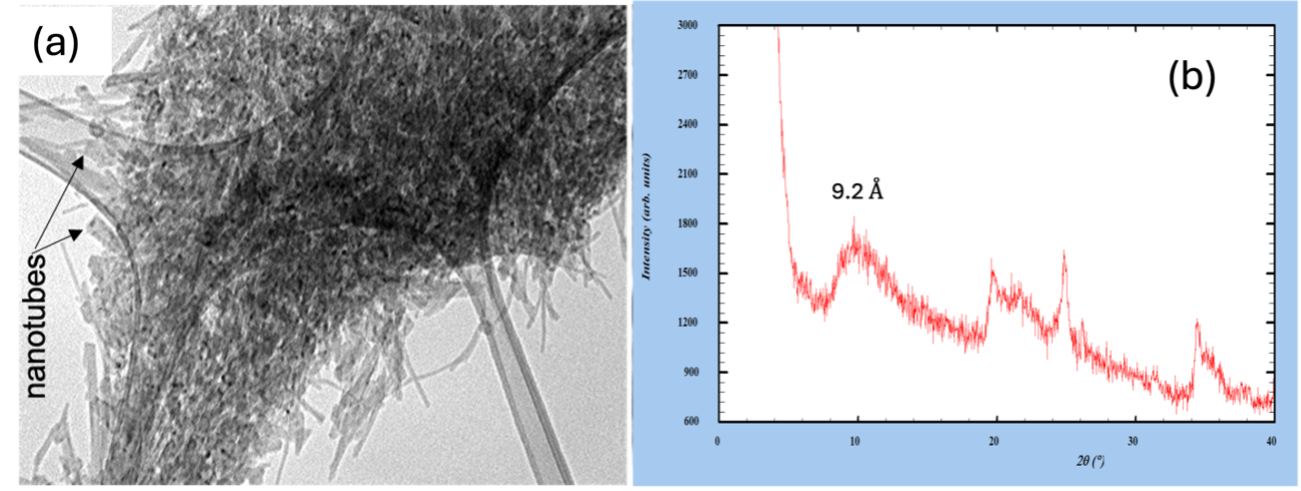
Figure 1. (a) Low-resolution TEM images showing nanotubular crystals compatible with halloysite. (b) XRD pattern of poorly crystallized halloysite intercalated with K-ac showing a characteristic peak at 9.2 Å.
References
[1] Benner, S. A. et al. 2000. PNAS, 97(6), 2425–2430.
[2] Lewis, J. M. T. et al., 2021. Journal of Geophysical Research: Planets, 126, e2020JE006803.
[3] Biemann, K., et al., 1976. Science, 194(4260), 72–76.
Acknowledgments
This research has been funded by the SOS-Mars (PID2020-119412RJ-I00) from MICINN Spain and the European Research Council CoG 818602.
How to cite: Gago-Duport, L., Losa-Adams, E., F. Bastero, S., G. Fairén, A., and Gil-Lozano, C.: Tracking the structural changes of poorly crystallized aluminosilicates by CheMin: A way to analyze the presence of organics in sediments of Gale Crater (Mars), Europlanet Science Congress 2024, Berlin, Germany, 8–13 Sep 2024, EPSC2024-1378, https://doi.org/10.5194/epsc2024-1378, 2024.