- 1LATMOS, Guyancourt, France (orianne.sohier@latmos.ipsl.fr)
- 2ENS Paris-Saclay, Gif sur Yvette, France
Context: Characterizing the atmospheres of small, temperate exoplanets addresses a major scientific challenge. The James Webb Space Telescope offers us the opportunity to study the atmospheric composition of temperate exoplanets as small as mini-Neptunes. Recent observations of K2-18b (Madhusudhan et al. 2023) and TOI-270d (Holmberg & Madhusudhan 2024; Benneke et al. 2024) have revealed atmospheres rich in hydrogen and carbon compounds. Methane and carbon dioxide have been detected in significant amounts. Moreover, the presence of haze is suggested by these observations (Madhusudhan et al. 2023), indicating that complex chemistry may be taking place in these atmospheres. However, our understanding of the chemistry governing these atmospheres remains largely unconstrained. Therefore, modeling and laboratory experiments are necessary to better understand these observations. Developing our knowledge of these atmospheres will help characterize their habitability.
1. Methods
Experimental Simulations: In this context, we carried out experimental simulations to reproduce the out-of-equilibrium chemistry that prevails in the upper layers of temperate exoplanets. We use a cold plasma reactor called PAMPRE (for Production d’Aérosols en Microgravité par Plasma REactif (Szopa et al. 2006)), a schematic diagram of which is shown in Figure 1. Our experimental procedure involves introducing into a reactive chamber selected gas mixtures at low pressure, analogous to the upper layers of the exoplanetary atmospheres of interest. The device then applies a radiofrequency discharge to the gas mixture, producing a plasma that triggers energetic processes. This experimental setup thus provokes an out-of-equilibrium chemistry similar to photodissociation intiated in the upper atmospheric layers exposed to energetic stellar particles and UV radiations. To track the chemical evolution in our experimental setup, we use mass spectrometry and infrared spectroscopy, enabling us to identify the formation of various chemical species.
Numerical Simulations: In parallel with our experimental work, we are carrying out numerical simulations using the ReactorUI code (Pernot 2023; Peng et al. 2014). ReactorUI was originally developed to simulate chemical reactions taking place in the reactor chamber in Titan’s anoxic N2/CH4 atmosphere. It has been updated for oxygenated reactions based on the chemical network of VULCAN, a 1D atmospheric simulation model (Tsai et al. 2017). This computational tool enables us to identify the chemical species produced in our experiments and elucidate the main chemical pathways leading to their formation. In addition, we compare VULCAN simulations to relate the results to expected atmospheric chemistry. This multi-faceted approach enables us to refine our understanding of the complex chemistry that occurs in the atmospheres of temperate exoplanets.
2. Results
Our experimentation highlights the production of complex organic compounds, as well as carbon monoxide and water vapour. We show that organic growth is favored in less oxidized environments, leading to the formation of long carbon chains, up to 6 carbons. Conversely, in more oxidized atmospheres, our initial results suggest the formation of oxidized organic compounds. Using infrared spectroscopy, we have identified a signature characteristic of carbonyls (C=O functional group) (Mobaraki & Hemmateenejad 2011), not associated with the signatures of CO2 or CO, as shown on Figure 2. This indicates a gas-phase oxidation reactions.
The first numerical simulations outputs are fairly consistent with the experimental ones. While VULCAN predicts for K2-18b atmosphere the presence of methanal (H2CO) and methanol (CH3OH) at 1mbar, with transport from the inner atmosphere making a significant contribution of thermodynamic equilibrium, ReactorUI confirms also the photochemical origin of these carbonyls and highlight the efficient formation of the simplest aldehyde, methanal, as shown on Figure 3.
Importantly, the volatility of organic compounds is reduced by these oxidation processes (Kroll & Seinfeld 2008), which may lead to their condensation in the atmospheric conditions prevailing on temperate exoplanets. These condensate species could serve as nucleation nuclei and contribute to the formation of haze or clouds (Zha et al. 2023).
Acknowledgements: N.C. thanks the European Research Council for funding via the ERC OxyPlanets projects (grant agreement No. 101053033)
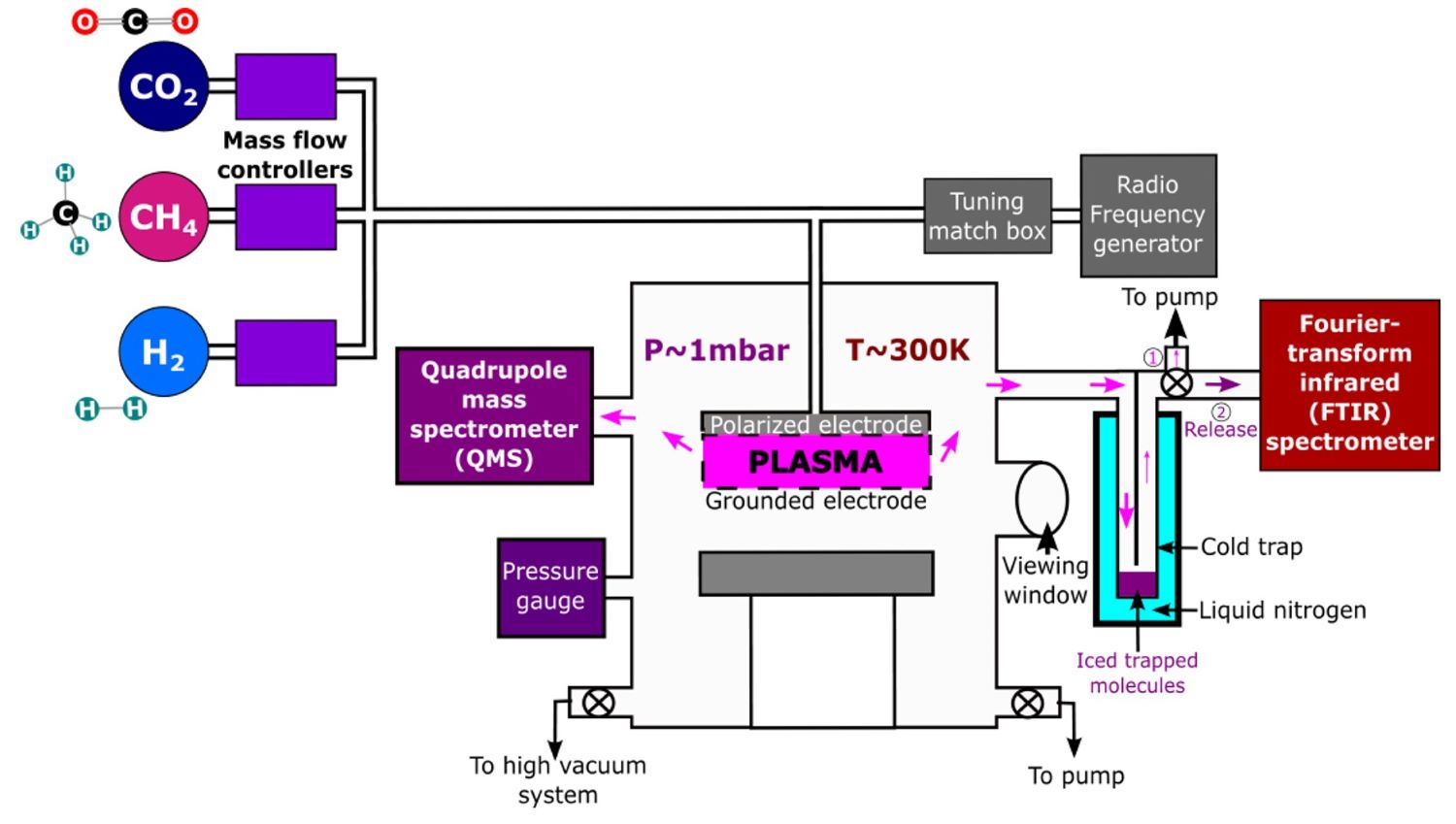
Figure 1: Schematic diagram of the PAMPRE setup. Selected gas mixtures are injected into a reactive chamber at 1 mbar and 300 K and ionized in a plasma.

Figure 2: Infrared spectra of gaseous mixtures of 98% H2 + 1% CH4 + 1% CO (left) and 90% H2 + 5% CH4 + 5% CO (right). In the most oxidized mixture (5% CO, right), the characteristic signature of carbonyl compounds (C=O) appears around 1700 cm−1.
Figure 3: Proportion of several species predicted by the ReactorUI code, for different gas mixtures. H2C=O formation is predicted with a relatively high abundance, in the ppm range (10−6).
References
Benneke, B., et al. 2024, JWST Reveals CH4, CO2, and H2O in a Metal-rich Miscible Atmosphere on a Two-Earth-Radius Exoplanet
Holmberg, M., et al. 2024, Possible Hycean conditions in the sub-Neptune TOI-270 d
Kroll, J. H., et al. 2008, Atmospheric Environment, 42, 3593, Chemistry of secondary organic aerosol: Formation and evolution of low-volatility organics in the atmosphere
Madhusudhan, N., et al. 2023, Carbon-bearing Molecules in a Possible Hycean Atmosphere
Mobaraki, N., et al. 2011, Chemometrics and Intelligent Laboratory Systems, 109, 171
Peng, Z., et al. 2014, GeoResJ, 1-2, 33, Modeling of synchrotron-based laboratory simulations of Titan’s ionospheric photochemistry
Pernot, P. 2023, ppernot/ReactorUI: Ready for sulfur
Szopa, C., et al. 2006, Planetary and Space Science, 54, 394, PAMPRE: A dusty plasma experiment for Titan's tholins production and study
Tsai, S.-M., et al. 2017, ApJ, 228, 2, VULCAN: An Open-source, Validated Chemical Kinetics Python Code for Exoplanetary Atmospheres
Zha, Q., et al. 2023, Oxidized organic molecules in the tropical free troposphere over Amazonia
How to cite: Sohier, O., Jaziri, Y., Vettier, L., Carrasco, N., Chatain, A., and Maratrat, L.: Understanding the chemistry of temperate exoplanets atmospheres: A study of oxidized organic compounds as precursors of photochemical condensates, Europlanet Science Congress 2024, Berlin, Germany, 8–13 Sep 2024, EPSC2024-218, https://doi.org/10.5194/epsc2024-218, 2024.