Habitability of the Martian Shallow Subsurface: Microbial Preference for Chlorate Over Perchlorate under Mars-like Conditions
- 1Technische Universität Berlin , Center for Astronomy and Astrophysics, RG Astrobiology, Germany
- 2GFZ German Research Center for Geosciences, Section Geomicrobiology, Potsdam, Germany
- 3Department of Plankton and Microbial Ecology, Leibniz-Institute of Freshwater Ecology and Inland Fisheries (IGB), Stechlin, Germany
The Martian surface and shallow subsurface lack stable liquid water, but hygroscopic salts in the regolith, including perchlorates and chlorates, can enable the transient formation of liquid brines. Hygroscopic salts such as perchlorates have been detected on Mars1, and the presence of chlorate salts is highly likely, as indicated by the detection of chlorate in the Martian meteorite EETA790012. Chlorate salts may be even more widespread on Mars, as recent experimental research suggests that under the hyperarid climate and the abundance of iron (hydro)oxide on Mars, chloride oxidation should yield significantly more chlorate than perchlorate3. Additionally, aqueous solutions on Mars may be more likely to be formed by chlorates than perchlorates, highlighting their importance for the habitability of Mars4. Perchlorate and chlorate salts can form liquid brines through a process called deliquescence, where the hygroscopic salt attracts water from the atmosphere to dissolve itself, or through the contact of these salts with water ice. In the shallow subsurface, a thin regolith layer can prevent water ice sublimation, allowing such liquid brines to persist for extended periods. Additionally, regolith layers can shield hypothetical microbes from harmful UV radiation, making the shallow subsurface a promising potential habitat for putative microbial life on Mars.
In this study, we investigated how the combined effects of (per)chlorate salts, UV irradiation, water scarcity, and regolith depth impact microbial survival under simulated Mars-like conditions. While previous studies have examined the effects of perchlorate-containing regolith and UV shielding on microorganisms, none have tested the impact of chlorate salts and regolith depths of multiple centimeters. Our Mars simulation experiments, conducted in the Mars Environmental Simulation Chamber (MESCH), described in detail by Jensen et al.5, uniquely allows for the simultaneous testing of increased salt stress due to water freezing at subzero temperatures and sublimation-induced desiccation at various sample depths. This is enabled by large sample tubes that accommodate regolith depths of up to 15 cm.
We exposed vegetative cells of Debaryomyces hansenii and Planococcus halocryophilus, and spores of Aspergillus niger, to simulated Martian environmental conditions (constant temperatures of about -11°C, low pressure of approximately 6 mbar, a CO2 atmosphere, and 2 hours of daily UV irradiation). Colony Forming Units (CFU) and water content were evaluated at three regolith depths (0-0.5 cm, 1-3 cm, 10-12 cm) before and after 3- and 7-day exposure periods. Each organism was tested under three conditions, where Mars regolith simulant was inoculated with cell suspensions of the three model organisms containing either: 1) 0.5 mol/kg NaClO3, 2) 0.5 mol/kg NaClO4, or 3) no additional salt. These conditions will, in the following sections, be referred to as NaClO3, NaClO4, and salt-free samples, respectively. In addition to the samples exposed to simulated Mars-like conditions, control samples of each organism, prepared in the same way as the exposure samples, were incubated for the same periods at -15°C in a freezer under normal Earth atmospheric conditions.
Our results showed that residual water content increased with depth in all three exposure experiments and for all three tested conditions. Remarkably, as illustrated in Figure 1, the survival rates of the organisms also increased with regolith depth in the NaClO3 and salt-free samples. However, survival rates in the NaClO4 samples were consistently lower across all depths, with the most significant difference observed at 10-12 cm, the depth with the highest residual water content. The proposed reason for this is the emergence of higher salt concentrations in the NaClO3 and NaClO4 samples due to the freezing of water retained in the regolith. This likely resembles realistic changes in brine concentrations in the Martian shallow subsurface. The higher survival rates in chlorate samples indicate that, for these organisms, perchlorate brines are more toxic than chlorate brines under the experimental conditions.Interestingly, in the NaClO4 samples, survival was higher at shallower depths. This can be linked to the shorter brine stability window at lower depths. Faster desiccation at lower depths prevents brines from persisting for long durations, minimizing the time salt stress is exerted on the organisms.
These findings, combined with the potential widespread occurrence of chlorate salts on Mars and their higher likelihood of forming liquid brines, highlight the need for further research on this oxychlorine species. Environments enriched with chlorate salts could be more habitable and should be considered in the search for microbial life on Mars, as most research has focused on the more toxic perchlorate salt.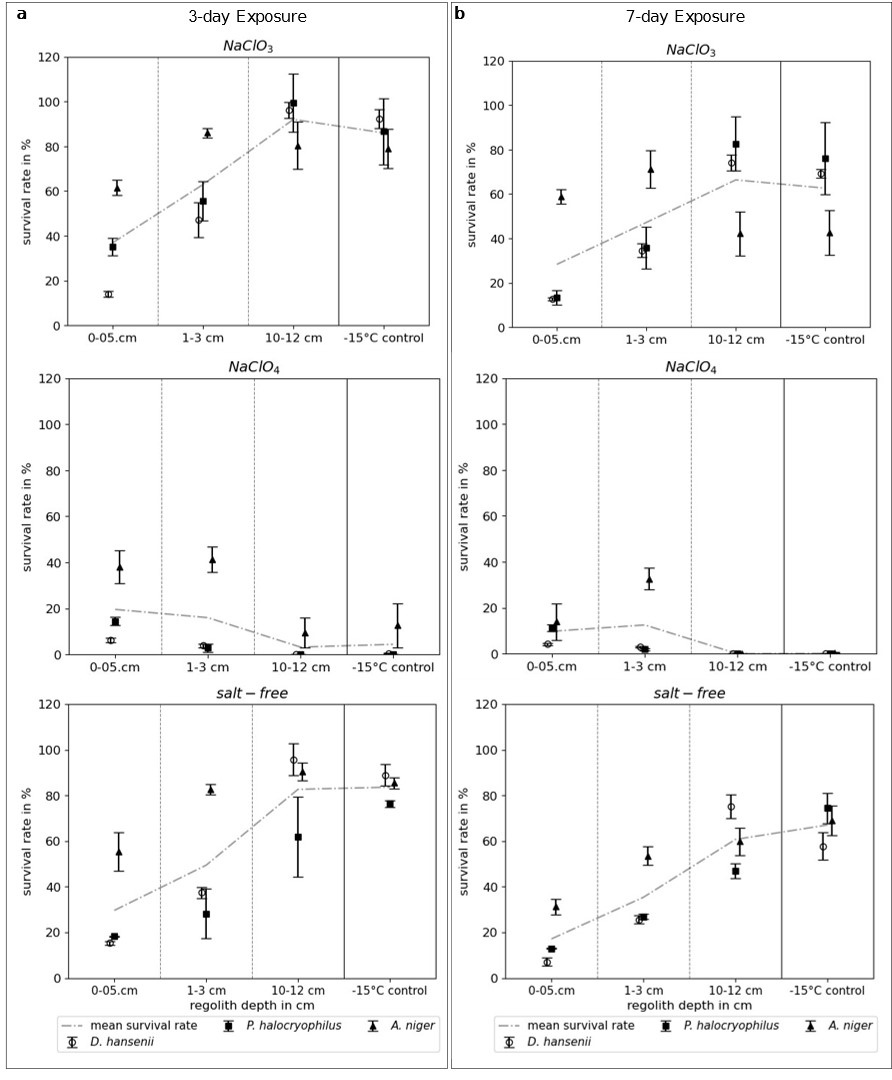
Figure 1: The median of the survival rates of D. hansenii, P. halocryophilus, A. niger (n=2, SE), and the mean survival rate (dashed line) of the three organisms after (a) a 3-day and (b) a 7-day exposure in the MESCH. The survival rates are displayed for three sample depths, as well as for the control incubated at -15°C in the freezer, in three tested conditions: NaClO3, NaClO4, and salt-free.
References:
1. Hecht, M. H. et al. Detection of Perchlorate and the Soluble Chemistry of Martian Soil at the Phoenix Lander Site. Science (80-. ). 325, 64–67 (2009).
2. Kounaves, S. P., Carrier, B. L., O’Neil, G. D., Stroble, S. T. & Claire, M. W. Evidence of martian perchlorate, chlorate, and nitrate in Mars meteorite EETA79001: Implications for oxidants and organics. Icarus 229, 206–213 (2014).
3. Qu, S.-Y. et al. Preferential Formation of Chlorate over Perchlorate on Mars Controlled by Iron Mineralogy. Nat. Astron. 6, 436–441 (2022).
4. Toner, J. D. & Catling, D. C. Chlorate brines on Mars: Implications for the occurrence of liquid water and deliquescence. Earth Planet. Sci. Lett. 497, 161–168 (2018).
5. Jensen, L. L. et al. A Facility for Long-Term Mars Simulation Experiments: The Mars Environmental Simulation Chamber (MESCH). Astrobiology 8, 537–548 (2008).
How to cite: Fischer, F. C., Schulze-Makuch, D., and Heinz, J.: Habitability of the Martian Shallow Subsurface: Microbial Preference for Chlorate Over Perchlorate under Mars-like Conditions, Europlanet Science Congress 2024, Berlin, Germany, 8–13 Sep 2024, EPSC2024-776, https://doi.org/10.5194/epsc2024-776, 2024.