- 1University of Leeds, School of Chemistry, Leeds, United Kingdom of Great Britain – England, Scotland, Wales (chmjeg@leeds.ac.uk)
- 2National Centre for Atmospheric Science, University of Leeds, Leeds, UK
- 3Met Office, Fitzroy Road, Exeter, UK
- 4School of Physics and Astronomy, University of Leeds, Leeds, UK
Understanding the composition and distribution of the unknown UV absorber (Figure 1) in the Venusian atmosphere has been an open question in planetary science for close to 100 years. Ferric chloride (FeCl3) has been proposed as the cause of the absorption [1], but the absorption spectrum of FeCl3 generally used in the literature was measured in ethyl acetate [2], which is not present on Venus and produces an absorption spectrum with little similarity to the Venusian absorber [3].

Figure 1: A false colour image of Venus. Regions of UV absorption appear orange. The cause of this absorption is unknown. Image credit: NASA/JPL-Caltech.
We have measured the absorption spectrum of FeCl3 in sulphuric acid with small quantities of HCl added, and we found that it is much more similar in shape to the observed spectrum of the unknown absorber than prior FeCl3 spectra available in the literature (Figure 2).
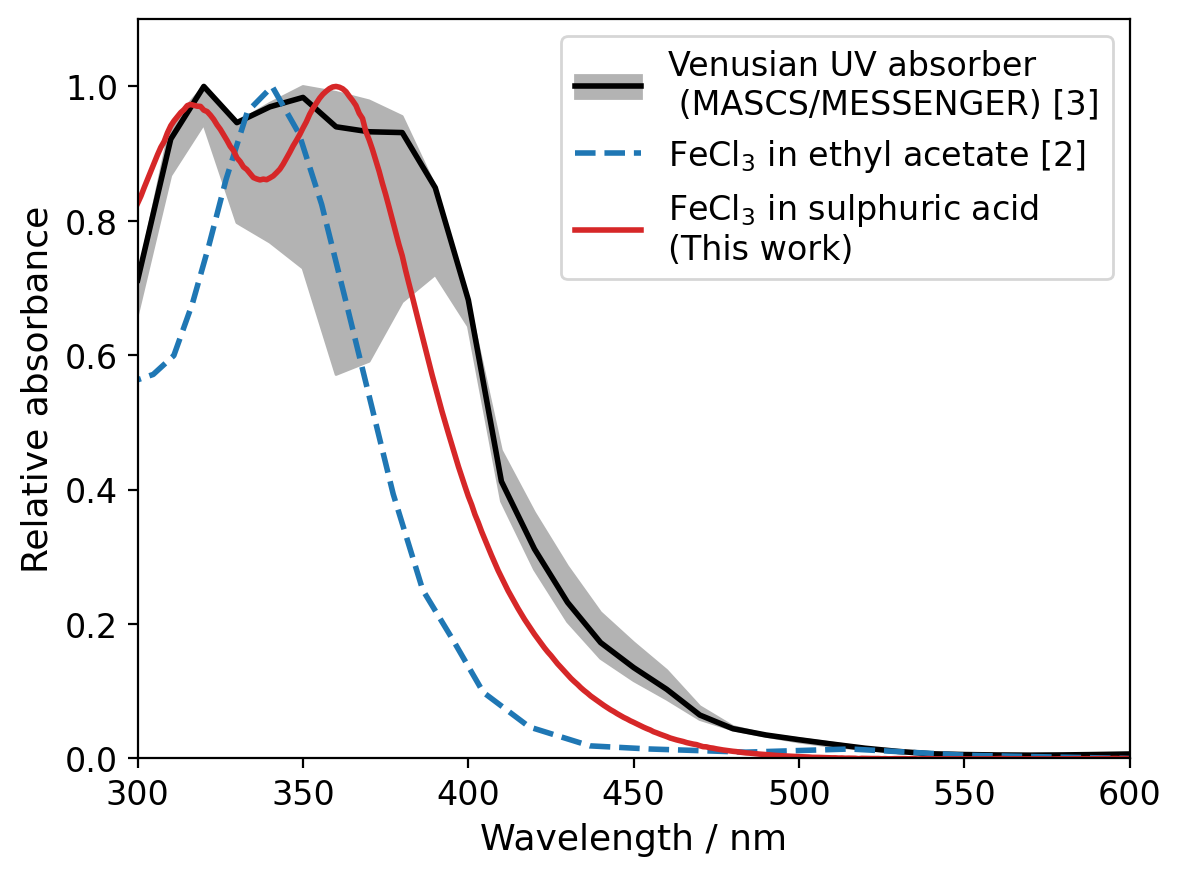
Figure 2: Comparison of the FeCl3 spectra in ethyl acetate [2] and sulphuric acid (this work) with a spectrum of the unknown UV absorber recorded by MASCS/MESSENGER [3].
When added to sulphuric acid, a mixture of ferric chloride and ferric sulphate ions are observed. We estimated the molar partitioning of the species in the mixtures by performing least squares fitting to reproduce each measured spectrum from spectra of pure ferric sulphate (measured for Fe2(SO4)3 in 75-78 wt% aqueous H2SO4) and pure ferric chloride (measured for FeCl3 in 5-37 wt% aqueous HCl).
The fraction of the observed absorption that can be reproduced by FeCl3 was investigated using three models: the global Planetary Climate Model for Venus (PCM-Venus) to model the photochemistry and 3D transport of the candidates in the atmosphere [4], a 1D sectional aerosol model to predict agglomeration and sedimentation as a transport mechanism of FeCl3 particles [5], and the 1D multiple scattering radiative transfer model SOCRATES [6].
The required concentrations of FeCl3 in the different cloud modes to reproduce observations taken by MESSENGER/MASCS during its June 2007 Venus flyby [3] were estimated for different cloud modes using SOCRATES. The full absorption can be explained by approximately 1.5 - 2 wt% FeCl3 in the mode 1 cloud droplets (Figure 3). Some contribution from ferric sulphate ions is also expected, but absorbs in the same wavelength region as SO2, so its precise contribution could not be reliable constrained.
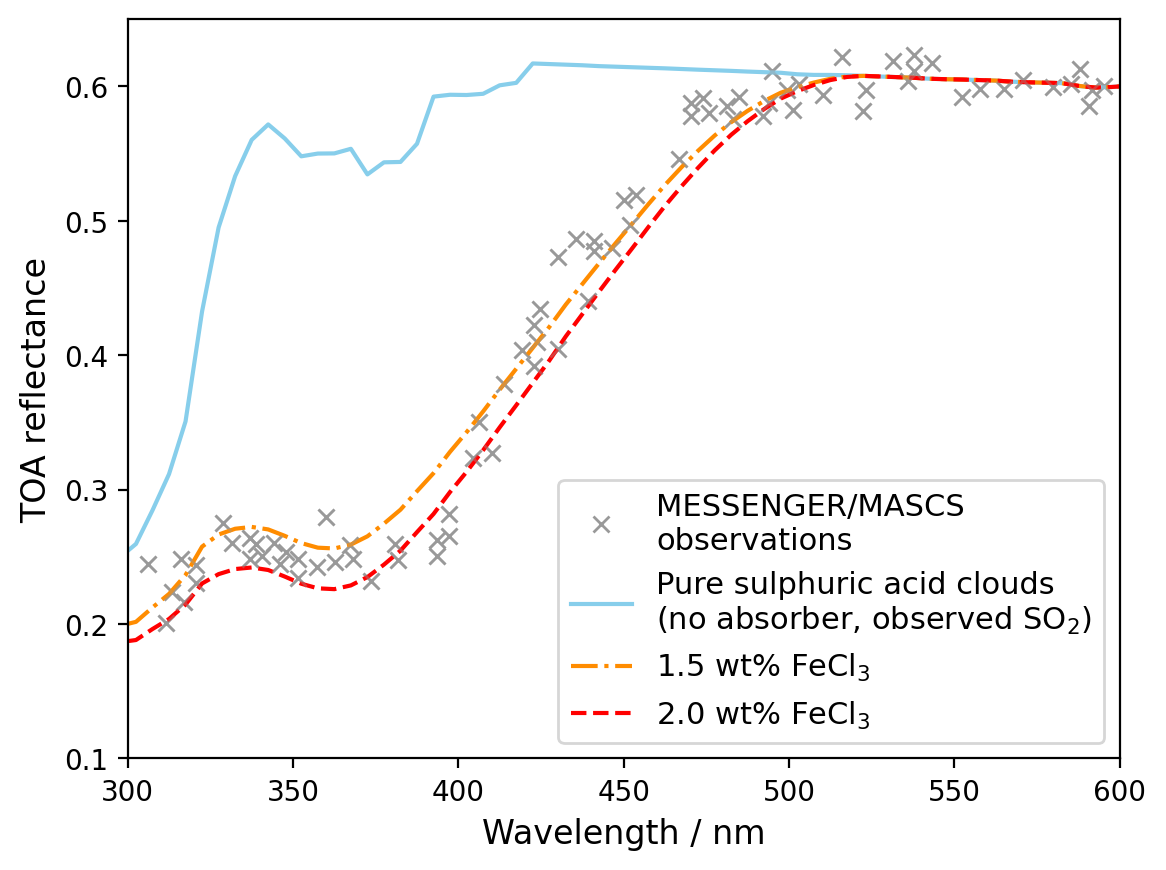
Figure 3: Comparison of MASCS/MESSENGER measured reflectance (crosses) [3] with the modelled spectrum with SO2 only (blue line), and with 1.2 – 2.0 wt% FeCl3 in mode 1 cloud droplets (orange and red lines).
Iron chemistry was added into PCM-Venus in order to predict the abundance of gas-phase FeCl3 produced by the reaction of gas-phase HCl with iron produced by the ablation of cosmic dust particles around 115 km. Mean gas and dynamical profiles from the PCM were used to initialise the agglomeration and sedimentation model, which was then run for many Venus years to reach steady state. Agglomeration and sedimentation modelling of FeCl3 suggests that the PCM-modelled FeCl3 column abundance above 60 km can account for more than 40% of the observed absorption. The flux of meteoric iron into the Venusian atmosphere has not been measured, and may be significantly higher than assumed in this work [7].
References
[1] Zasova et al. 1981, https://doi.org/10.1016/0273-1177(81)90213-1
[2] Aoshima et al. 2013, http://doi.org/10.1039/C3PY00352C
[3] Pérez-Hoyos et al. 2018, https://doi.org/10.1002/2017JE005406
[4] Martinez et al. 2024, doi.org/10.1016/j.icarus.2024.116035
[5] Frankland et al. 2017, https://doi.org/10.1016/j.icarus.2017.06.005
[6] Manners et al. 2022, SOCRATES Technical Guide, available at: https://code.metoffice.gov.uk/trac/socrates
[7] Carrillo-Sánchez et al. 2020, https://doi.org/10.1016/j.icarus.2019.113395
How to cite: Egan, J., Feng, W., James, A., Manners, J., Marsh, D., and Plane, J.: Investigation of ferric chloride as the cause of the Venusian NUV absorption, EPSC-DPS Joint Meeting 2025, Helsinki, Finland, 7–12 Sep 2025, EPSC-DPS2025-1039, https://doi.org/10.5194/epsc-dps2025-1039, 2025.