- 1California State Polytechnic University, Pomona
- 2Blue Marble Space
- 3Arizona State University
- 4NASA Goddard Institute for Space Studies
- 5University of Wisconsin, Madison
Introduction
In this work, we describe new and transformative insights into the composition of Venus’ aerosols using uncharacterized data acquired by the Pioneer Venus Large Probe (PVLP), which descended through Venus’ atmosphere in 1978 [1]. During the descent, the PVLP inadvertently collected cloud aerosols and measured the decomposition of their contents [2]. In the initial post-flight PVLP investigations, aerosol collection and analysis were inferred for the Large Probe Neutral Mass Spectrometer (LNMS) [2] and considered for the Large Probe Gas Chromatograph (LGC) [3].
Here, we demonstrate that the LNMS data contain several uncharacterized mass signals, which directly relate to the thermal decomposition of aerosols possessing a heterogeneous chemical composition. Accordingly, we re-analyzed and re-interpreted the altitude trends from the LNMS data and LGC results as the evolved gas analyses that followed the temperature gradient of the PVLP descent (-30 to 462 ˚C, ~ 65 to 0 km [4]).
Methods
Thermal decomposition profiles from the LNMS data were constructed by plotting the number densities for SO2, H2O, SO3, and O2 against the LNMS inlet temperatures, which were heated during operation. The LNMS inlet temperatures were obtained using data from the LNMS project reports [5] and PVLP descent timeline [6]. The mass spectra obtained at the electron ionization energies of 70, 30, and 22 eV were independently treated using the procedures described in [7-9]. The peaks for SO2, H2O, SO3, and O2 in the thermal profiles were fit to Gauss functions and the regressions minimized by least squares analysis. Using the stoichiometry in Reactions 1-3, the peak areas for SO2 and H2O were converted to weight percent (w%) and mass loading (mg m-3) for H2SO4, Fe2(SO4)3, and H2O (after correction for the H2O from Reaction 1). Review of the LNMS CO2 density profile [9] revealed that aerosols were collected between ~ 51.0 to 48.4 km. The collection column was treated as a cylinder with a height of 2.6 km and diameter of 0.98 m, which equaled the outer PVLP diameter [10].
H2SO4 → SO3 + H2O (1)
SO3 → SO2 + 0.5 O2 (2)
Fe2(SO4)3 → Fe2O3 + 3 SO3 (3)
Results and Discussion
The LNMS mixing ratios (x) for SO2 and H2O (Fig. 1) are inconsistent with other Venus measurements obtained spectroscopically. Yet, after aerosol capture (< 51.0 km, Fig. 1), the LNMS and LGC xSO2 and xH2O are within error (or comparable). Moreover, the cloud xH2O from the LNMS are lower than other Venus measurements obtained by direct analyses (Fig. 1). These combined results suggest that the xSO2 and xH2O from the LNMS and LGC do not represent atmospheric gases. Instead, the LNMS and LGC xSO2 and xH2O the represent the gases released during the thermal decomposition of the captured aerosols. The differing maxima observed in the LNMS xSO2 and xH2O (at ~ 35 and 10 km) suggest the decomposition of ≥ 2 sulfate-bearing compounds from the captured aerosols.
Re-expression of the LNMS data as evolved gas profiles (Fig. 2) revealed the temperature-dependent release of SO2, H2O, SO3, and O2 from the captured aerosols. Parsing of the LNMS data additionally revealed signals consistent with the release of metal-bearing species (e.g., FeO+ and MgSO4+). These results are consistent with an aerosol composition including H2SO4, hydrated ferric sulfates, possibly hydrated magnesium sulfate, and other hydrates. The thermal decomposition reactions for H2SO4 and ferric sulfates are provided in Reaction 1-3. The inferred aerosol relative abundances amounted to 22 ± 4 wt% H2SO4, 16 ± 3 wt% Fe2(SO4)3 (projected), and 62 ± 8 wt% H2O. The aerosols H2O predominantly released from hydrates between ~ 270–460 ˚C (59 ± 8 wt%), consistent with the loss of H2O from hydrated iron and magnesium sulfates and other hydrates (e.g., [11, 12]).
Thus, this work reveals that Venus’ aerosols contain a previously underestimated reservoir of water and possible altered materials derived from iron and magnesium of cosmic origin. These results provide new considerations for cloud chemistry models and cloud habitability discussions.
Figure 1. The LNMS apparent mixing ratios for (A) SO2 and (B) H2O compared to the LGC and other Venus measurements. Brackets to the right side of the plots indicate where the differing aerosol components decomposed. The LNMS values (squares) were obtained from the spectra obtained at 70 eV (red squares) and 30 and 22 eV (green squares). The LGC (yellow circles) error bars represent the reported 3 s confidence intervals. The comparative Venus values (green triangles) for (A) SO2 and (B) H2O are respectively labeled in the top legends. Pertinent abbreviations include NIR (near infrared spectroscopy), GC (gas chromatograph), UVS (UV spectroscopy), V12 (Venera 12), V13/14 (Venera 13 and 14), and V11/13/14 (Venera 11, 13, and 14).
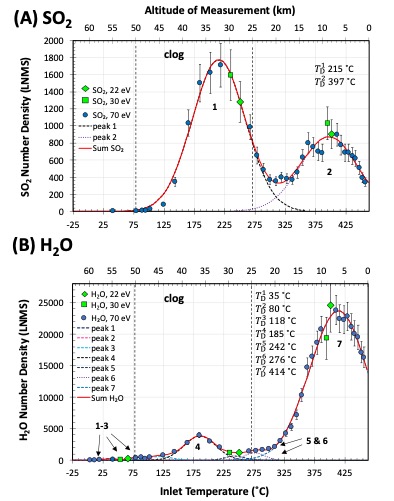
Figure 2 The LNMS evolved gas profiles for (A) SO2 and (B) H2O expressed against the LNMS inlet temperatures (lower x-axis) and associated altitudes (upper x-axis). Number densities are from the 70 (circles), 30 (squares), and 22 eV (diamonds) spectra. Fits to the LNMS data (solid red lines) represent the sum of the numbered Gauss peaks (dashed lines). Temperatures at maximum decomposition (TD) for each peak (n) are listed. Dotted lines demark the LNMS clog (~ 50-25 km).
References
[1] Fimmel R. O. Pioneer Venus (1983) Scientific and Technical Information Branch, NASA.
[2] Hoffman J. H. et al. (1980) JGR Space Sci., 85, 7882-7890.
[3] Oyama V. I. et al. (1979) Science, 205, 52-54.
[4] Seiff A. et al. (1985) Adv. Space Res., 5, 3-58.
[5] Final Report, Large Probe Neutral Mass Spectrometer, August 31, 1978, NASA Ames Research Center History Archives, Collection Number AFS8100.15A.
[6] Seiff A. et al. (1980) JGR Space Sci., 85, 7903-7933.
[7] Mogul R. et al. (2021) GRL, 48, e2020GL091327.
[8] Mogul R. et al. (2023) Icarus, 392, 115374.
[9] Mogul R. et al. (2023) MethodsX, 11, 102305.
[10] Dutta S. et al. In AIAA SCITECH 2023 Forum (2023), 1165.
[11] Lauer Jr H. et al. In 31st Lunar and Planetary Science (2000), 20000085925.
[12] Spratt H. et al. (2014) J. Therm. Analysis Calorim., 115, 101-109.
How to cite: Mogul, R., Zolotov, M., Way, M., and Limaye, S.: Iron sulfate, sulfuric acid, and water are major components in Venus’ aerosols , EPSC-DPS Joint Meeting 2025, Helsinki, Finland, 7–12 Sep 2025, EPSC-DPS2025-1089, https://doi.org/10.5194/epsc-dps2025-1089, 2025.