- 1Laboratoire Atmosphères, Observations Spatiales (LATMOS), Université Versailles Saint-Quentin, UPMC Université Paris 06, CNRS, LATMOS, Guyancourt, France
- 2Laboratoire de Génie des Procédés et Matériaux (LGPM), CentraleSupélec, Gif-sur-Yvette, France
1. Introduction
Mars’ surface is currently one of the environments in the solar system, where the research about past prebiotic chemistry is the more active, because Mars gathered the conditions required for the emergence of life at the time it arose on Earth (3.7-4 Ga) [1].
In this context, the Curiosity rover landed in Gale crater in 2012. This ancient lake shows stratified geological units which favors the study of different periods in the history of the crater. Onboard the Curiosity rover, a gas chromatograph mass spectrometer (GCMS) instrument, part of Sample Analysis at Mars (SAM) experiment, has the objective to detect and identify organic and inorganic molecules in surface samples collected by the rover. In the recent years, Curiosity has explored a strata enriched in sulfates, such as magnesium sulfates or iron sulfates, on its ascent of Mount Sharp [2]. Like other minerals and inorganic phases, sulfates may contain organic matter and protect it from the harsh surface environmental conditions. Likewise, sulfates can play a role in the chemical extraction of organic matter by the sample preparation techniques used by SAM, i.e. pyrolysis, chemical derivatization and thermochemolysis [3]. To support the treatment and the interpretation of the data provided by SAM, it is of primary importance to perform laboratory experiments mimicking the sample treatments and operating conditions used by SAM.
In this frame, we performed a systematic analysis of a variety of synthetic and natural samples containing both sulfates and organic molecules by reproducing the SAM analytical conditions, with a specific interest for magnesium and iron sulfates detected in Gale crater.
2. Samples and experimental set up
Considering the complexity of natural samples to infer possible chemical interactions between organic molecules and sulfates that may induce the production of detected S-bearing compounds [3][4], a first set of synthetic samples was made and studied. The samples were made by mixing individual sulfates and organic molecules representative of chemical species either suspected to be present on Mars or of interest for astrobiology. These results were then compared with natural Martian analogs. For the synthetic samples, undecanoic acid and benzoic acid were selected because both of them are possible precursors of molecules detected by SAM in Cumberland samples [5][6]. Naphthalene was selected as a possible molecule delivered by meteoritic influx and valine as an amino acid of interest for astrobiology. Regarding the sulfate phase, Fe-sulfate and Mg-sulfate have been used because they were both detected in Gale crater with different instruments onboard Curiosity. Typical synthetic samples were made by mixing the organic compound at 10 wt% ratio in sulfates in aquese phase.
Samples analyses were performed with a laboratory set up simulating the analytic pathway and operating condition of SAM, based on a gas chromatograph mass spectrometer coupled with an oven pyrolyzer (figure 1). This last one allows to reproduce the pyrolysis conditions of the SAM instrument. The pyrolyser ramp up to 850 °C with a ramp of 35 °C min-1 [7]. The gases released by the sample are then trapped and focused at the GC column inlet cooled with liquid nitrogen during the whole duration of the pyrolysis. The gases are then quickly released to the chromatograph by stopping the cryocooling. Finally, to complete the scope of this study, the samples were also analyzed using the two other sample preparation techniques used in SAM, i.e., thermochemolysis with tetramethylammonium hydroxide (25% in methanol), and derivatization with a 4:1 mix of N,N-methyltert-butyl-dimethylsilyltrifluoroacetamide:N,N-dimethylformamide. In this last case the reaction was done ex situ and the resulting reaction mixture was injected as a liquid sample directly into the GC using a syringe injection.
Figure 1: Schematic of the experimental pathway from the pyrolysis of the sample to the detection of the molecules
3. Results
The first results obtained by the pyro-GCMS reveal interactions between sulfate and organic compounds during the pyrolysis by the production of S-bearing compounds or sulfurization of the organic molecule, except in the case of naphthalene.
As an example of results, laboratory experiments done with a mixture of undecanoic acid and Mg-sulfate, using the SAM-like pyrolysis conditions, show two important features. First, when pyrolyzed at 850°C in presence of sulfates, undecanoic acid seems more inclined to create aromatic compounds. This could result from the acid being trapped in the sulfate’s crystal and being released at high temperature resulting in a cyclisation of the acid. Second, the acid does react with the salt to produce, especially, thiophene based compounds (figure 2).
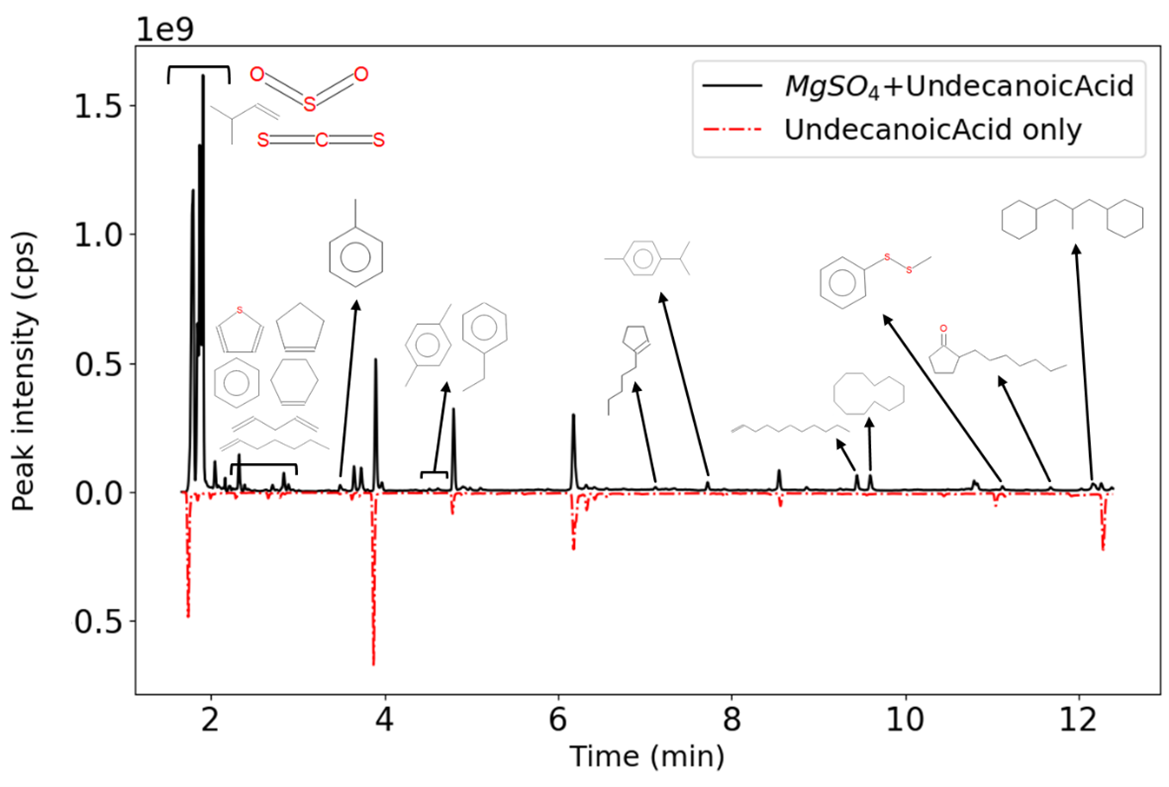
Figure 2: Chromatogram obtained from GCMS analysis of undecanoic acid after a pyrolysis at 850 °C, with a heating ramp of 35 °C min-1. The molecules represented on the upper chromatogram were found in presence of magnesium sulfates. The control using undecanoic acid alone is display on the lower chromatogram.
It is also interesting to notice, that with and without sulfates, undecanoic acid is degraded in smaller alkanes and oxidized up to CO2. As a consequence, small alkane chains, aromatic molecules, and thiophene based compounds detected from those analyses are coherent with SAM detection and may be a clue to understand the interaction of aliphatic carboxylic acid with sulfates.
In this presentation, we will give an overview of the results obtained in this study for all the samples, and we will conclude on the consequences for the results obtained with the SAM experiment.
4. Acknowledgements
SAM-GC team acknowledges support from the French Space Agency (CNES), National French Council (CNRS), and DIM ORIGINS of Région Ile de France.
References
[1] Wordsworth, R. D. (2016). Annual Review of Earth and Planetary Sciences, 44, 381-408.
[2] Sutter et al., (2017). Journal of Geophysical Research: Planets, 122(12), 2574-2609.
[3] Millan et al., (2022). Journal of Geophysical Research: Planets, 127(11), e2021JE007107.
[4] Eigenbrode et al., (2018). Science, 360(6393), 1096-1101.
[5] Freissinet et al., (2015). Journal of Geophysical Research: Planets, 120(3), 495–514.
[6] Freissinet et al., (2025). Proceedings of the National Academy of Sciences, 122(13), e2420580122.
[7] Mahaffy et al., (2012). Space Science Reviews, 170, 401-478.
How to cite: Govekar, T., Szopa, C., Freissinet, C., Millan, M., Buch, A., and Boulesteix, D.: Analysis of organic molecules in the presence of sulfates with gas chromatography mass spectrometry to interpret Curiosity data, EPSC-DPS Joint Meeting 2025, Helsinki, Finland, 7–12 Sep 2025, EPSC-DPS2025-1356, https://doi.org/10.5194/epsc-dps2025-1356, 2025.