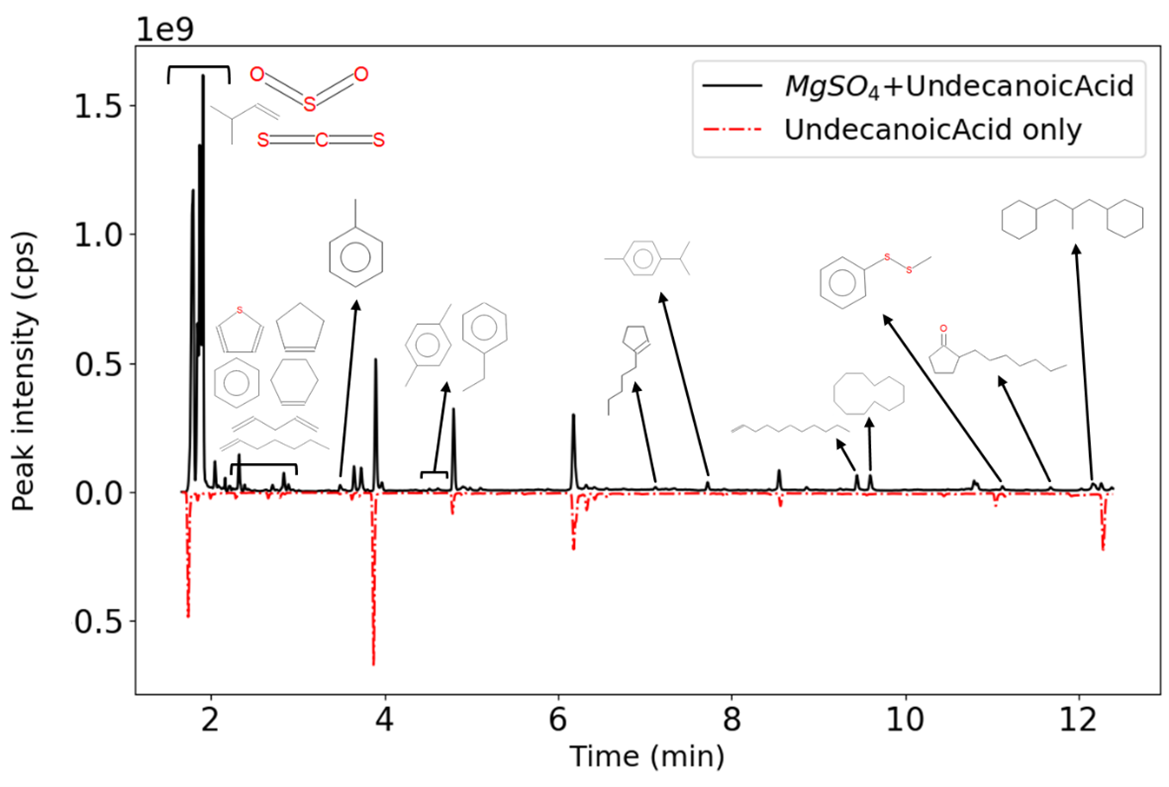
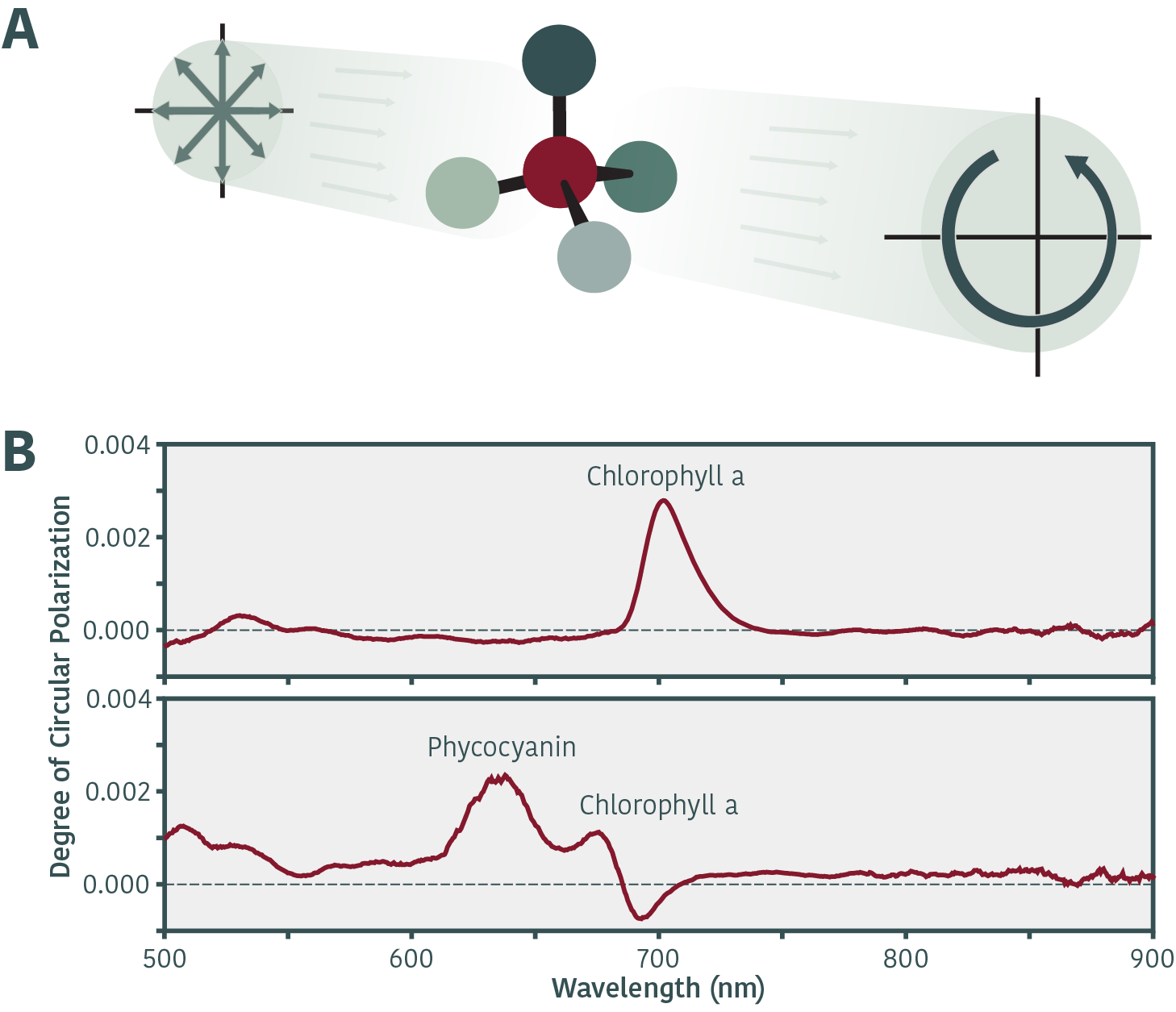
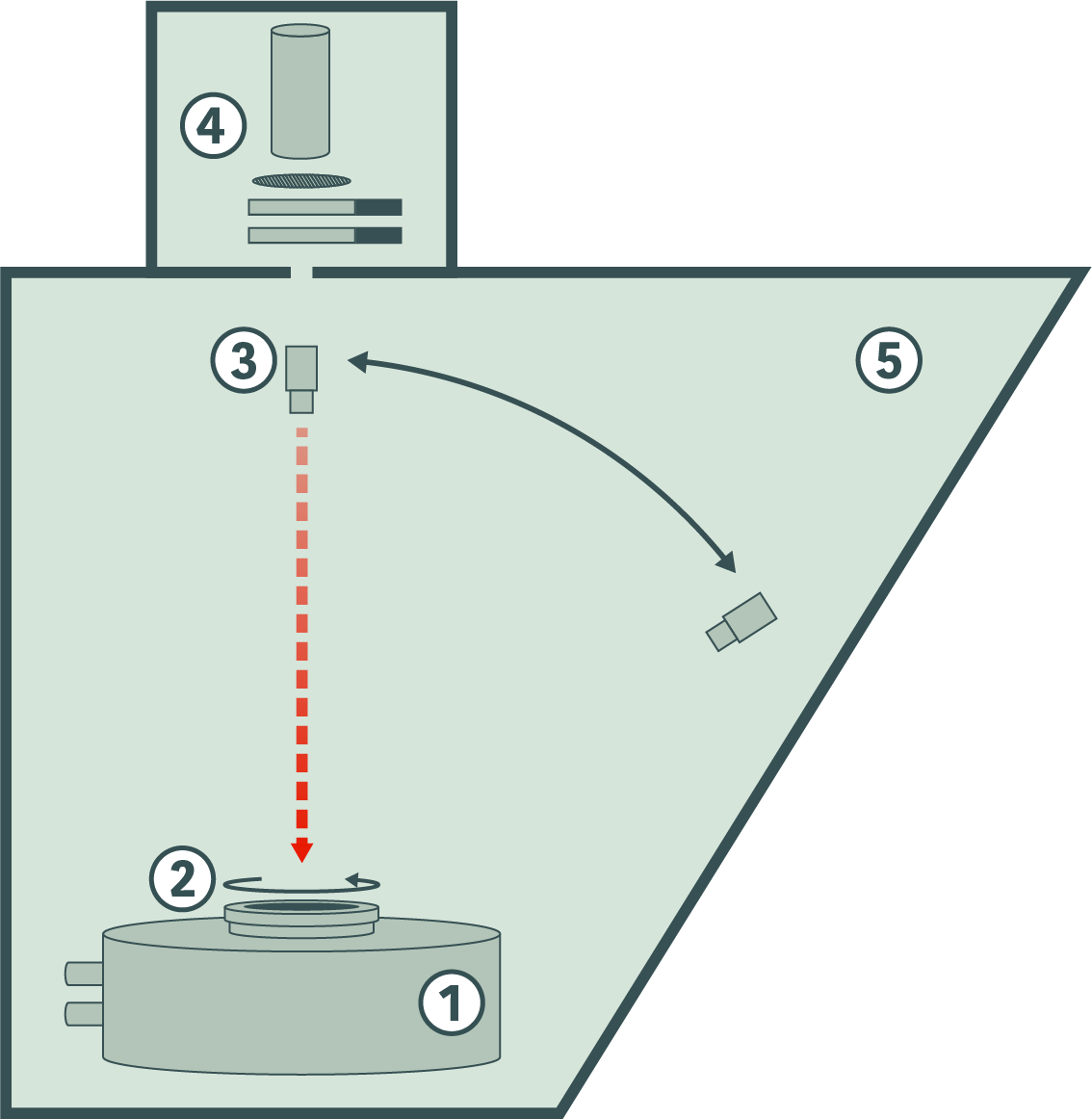
EXOA7
Astrobiology
Orals TUE-OB6
|
Tue, 09 Sep, 16:30–17:54 (EEST) Room Venus (Veranda 3)
Posters TUE-POS
|
Attendance Tue, 09 Sep, 18:00–19:30 (EEST) | Display Tue, 09 Sep, 08:30–19:30 Finlandia Hall foyer, F212–220
Understanding how the planetary environment has influenced the evolution of life and how biological processes have changed the environment is an essential part of any study of the origin and search for signs of life. A central issue in the research on the emergence of life is the paradoxical role of water in pre-biotic chemistry. In fact,on the one hand, water is essential for all known life, on the other hand it is highly destructive for key biomolecules such as nucleic and polypeptides. Earth analogues experiments/instruments test and/or simulation campaigns and limits of life studies are included as well as one of the main topics of this session.
Major Space Agencies identified planetary habitability and the search for evidence of life as a key component of their scientific missions in the next two decades. The development of instrumentation and technology to support the search for complex organic molecules/sings of life/biosignatures and the endurance of life in space environments is critical to define unambiguous approaches to life detection over a broad range of planetary environments. A truly interdisciplinary approach is needed to delve into the core of the issue of emergence of life, because in addition to physics and chemistry it is also need to deploy a number of other sciences. We rely on contribution coming from mathematical or philosophical perspectives not only on astrobiology moreover we think that a part of the answers may lie in scientists who working on cancer research, genetics, space exploration paleontology who are not necessarily involved in this field.
Session assets
16:30–16:42
|
EPSC-DPS2025-131
|
ECP
|
On-site presentation
16:42–16:54
|
EPSC-DPS2025-192
|
ECP
|
On-site presentation
16:54–17:06
|
EPSC-DPS2025-765
|
On-site presentation
17:06–17:18
|
EPSC-DPS2025-940
|
ECP
|
On-site presentation
17:18–17:30
|
EPSC-DPS2025-1971
|
On-site presentation
17:30–17:42
|
EPSC-DPS2025-218
|
ECP
|
On-site presentation
17:42–17:54
|
EPSC-DPS2025-1512
|
ECP
|
On-site presentation
F212
|
EPSC-DPS2025-68
|
Virtual presentation
F213
|
EPSC-DPS2025-121
|
ECP
|
On-site presentation
F214
|
EPSC-DPS2025-389
|
ECP
|
On-site presentation
F215
|
EPSC-DPS2025-441
|
On-site presentation
F216
|
EPSC-DPS2025-1237
|
ECP
|
On-site presentation
F217
|
EPSC-DPS2025-1356
|
ECP
|
On-site presentation
F218
|
EPSC-DPS2025-1799
|
ECP
|
On-site presentation
F219
|
EPSC-DPS2025-716
|
ECP
|
On-site presentation
F220
|
EPSC-DPS2025-1749
|
ECP
|
On-site presentation
Experimental Simulation of Europan Seafloor Hydrothermal Systems
(withdrawn)