- 1University of Helsinki, Department of Physics, Helsinki, Finland (giorgia.incaminato.02@gmail.com)
- 2Istituto di Astrofisica e Planetologia Spaziali, INAF-IAPS, Rome, Italy
- 3Institute of Planetary Research, Deutsches Zentrum für Luft und Raumfahrt, Berlin, Germany
- 4Laboratory of Photonics, Tampere University, Finland
- 5Laboratory of Physical Chemistry, Department of Chemistry, University of Helsinki, Finland
It is of crucial importance to gain a more profound comprehension of the evolution and formation of Mercury, one of the terrestrial planets in the Solar System. The absence of a significant atmosphere, temperature oscillations, and the continuous exposition to solar wind, result in Mercury surface being a mixture of crystalline and glassy materials (Wurz et al., 2025).
The particularity about the Mercury surface is that it has a remarkably low reflectance, but NASA’s MESSENGER mission did not detect the absorption band of iron in the NIR, implying the iron content on Mercury surface would be low compared to other dark planetary bodies (Syal et al. 2015). However, observations and modelling suggest that a darkening agent is needed to explain the low reflectance in the Vis-NIR spectra of Mercury surface. The agent is thought to be carbon, particularly in the form of graphite (Lark et al. 2023).
We proposed to test if introducing carbon would darken the Mercury surface analogue materials to the desired level. To investigate this, the material UV-Vis-NIR spectral reflectances were analysed. We prepared three types of samples: “St” glass externally mixed with soot (Figure 1), 90NaPO3-10NaF (mol%) glass (Figure 2), and komatiite (volcanic glass 12%, olivine 35%, clinopyroxene 15%, plagioclase 21%, spinel 9%, opaques 4%, and serpentine 3%) to which different amounts of graphite up to 7.5-wt% were added into the glass batch prior to the melting. Komatiite is recognized as a good analogue for the Mercury surface (Caminiti et al. 2024; Wieder et al. 2012). The phosphate glass was melted at 750°C, whereas komatiite was melted at 1600°C.
Analysis of the UV-Vis-NIR reflectance spectra of the komatiite glass revealed that graphite did not survive the melting process. The undoped komatiite showed the lowest reflectance and increasing the initial graphite content resulted in a brighter, rather than darker glass (Figure 3). High temperature and the presence of atmospheric oxygen in the furnace probably led to its oxidation, releasing it as CO or CO2.
To qualitatively evaluate the surface properties of the grains and determine with precision the cause of what is suggested by the reflectance spectra of the komatiite intimately mixed with graphite, Scanning Electron Microscopy (SEM) was performed. The SEM analysis showed a progressive change in size, shape, and roughness of the grains with the increase of graphite initially added, with a direct correlation between their morphological irregularity and the graphite content used in the melting process of komatiite, which directly affect the optical properties of the material, leading to a higher reflectance for the komatiite powder with an initially higher -wt% of graphite. To evaluate the effect of graphite also on the komatiite, graphite was added externally in the same -wt% as previously. The reflectance spectra show that, when graphite is externally added, its effect is in line with the expectations for decreasing reflectance with increasing concentration (Figure 4).
Our study confirms that graphite is an effective darkening agent and could plausibly contribute to the low reflectance of the Mercury surface. The main challenge has been the melting of the glass in an oxygenated environment, so future work will focus on replicating the melting process in an oxygen-free atmosphere.
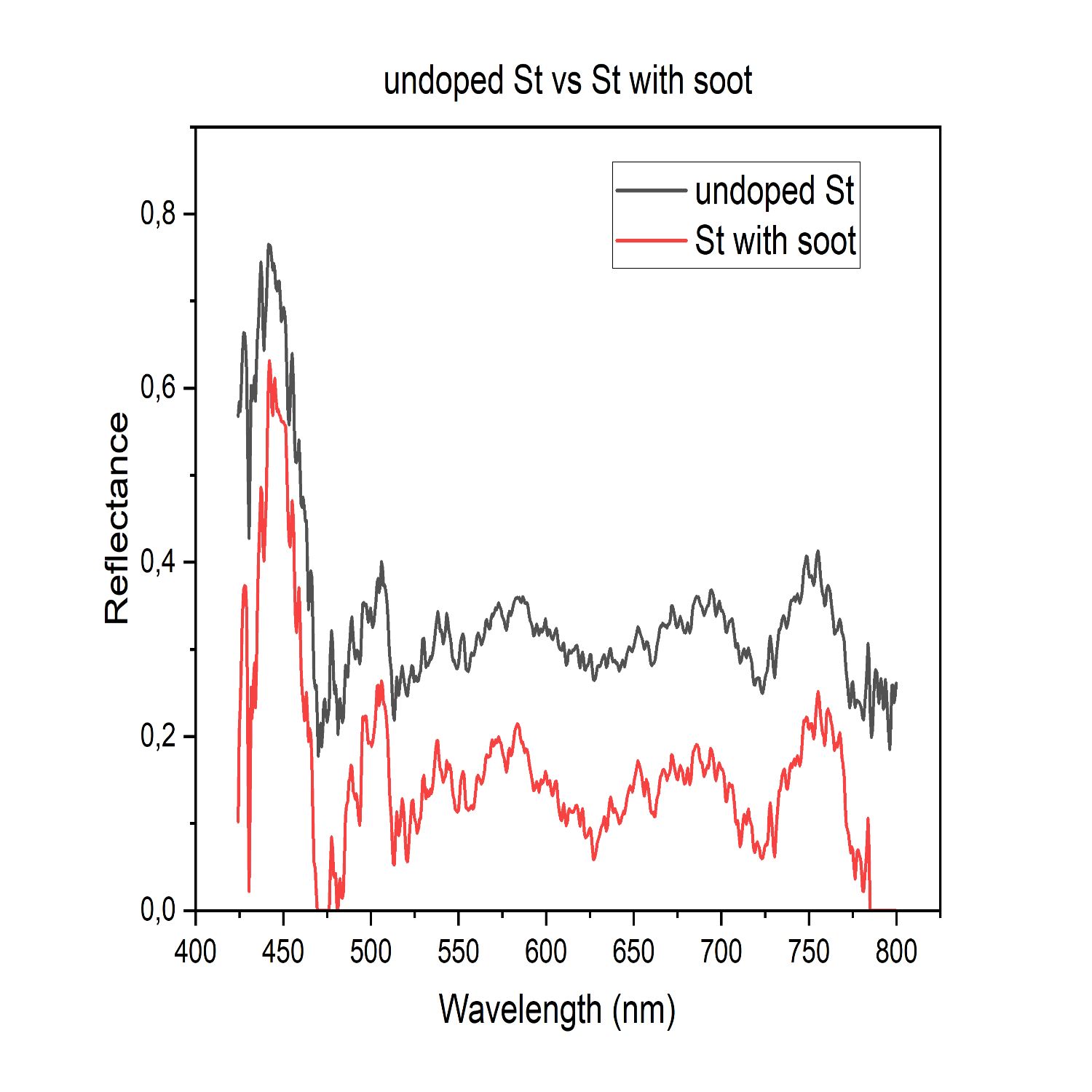
Figure 1: Spectral reflectance of the St glass powders. In black the reflectance spectrum of the undoped St glass powder; in red the reflectance spectrum of the St glass powder externally doped with 0.05 wt% of soot.
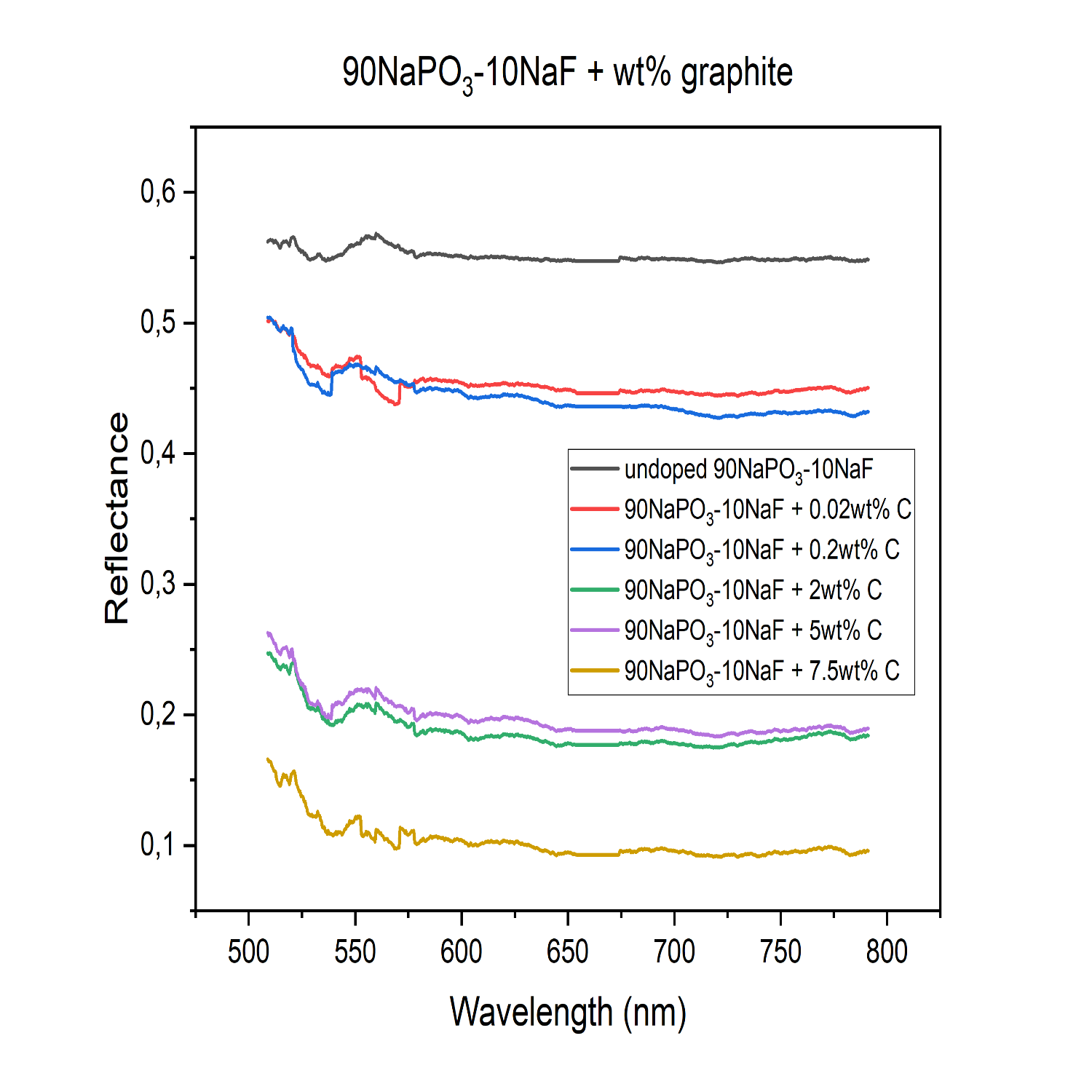
Figure 2: Spectral reflectance of the 90NaPO3-10NaF (mol%) glasses with various wt% of graphite, designated as C, intimately added. As the amount of graphite added increases, reflectance decreases.
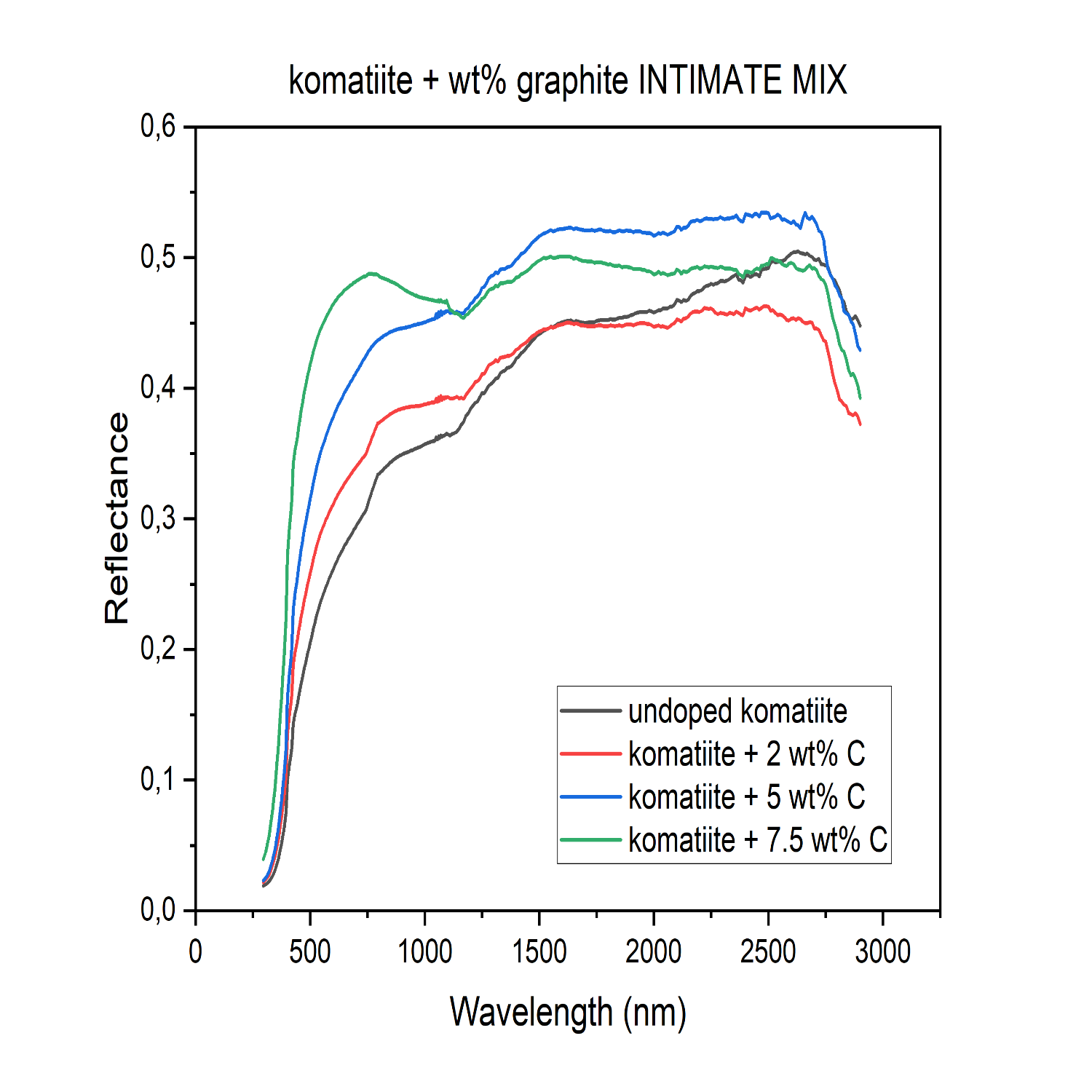
Figure 3: Spectral reflectance of the komatiite glasses with various wt% of graphite, designated as C, intimately added. The spectra reveal that, as the amount of graphite initially added to the glass composition prior to the melting increases, the reflectance increases.
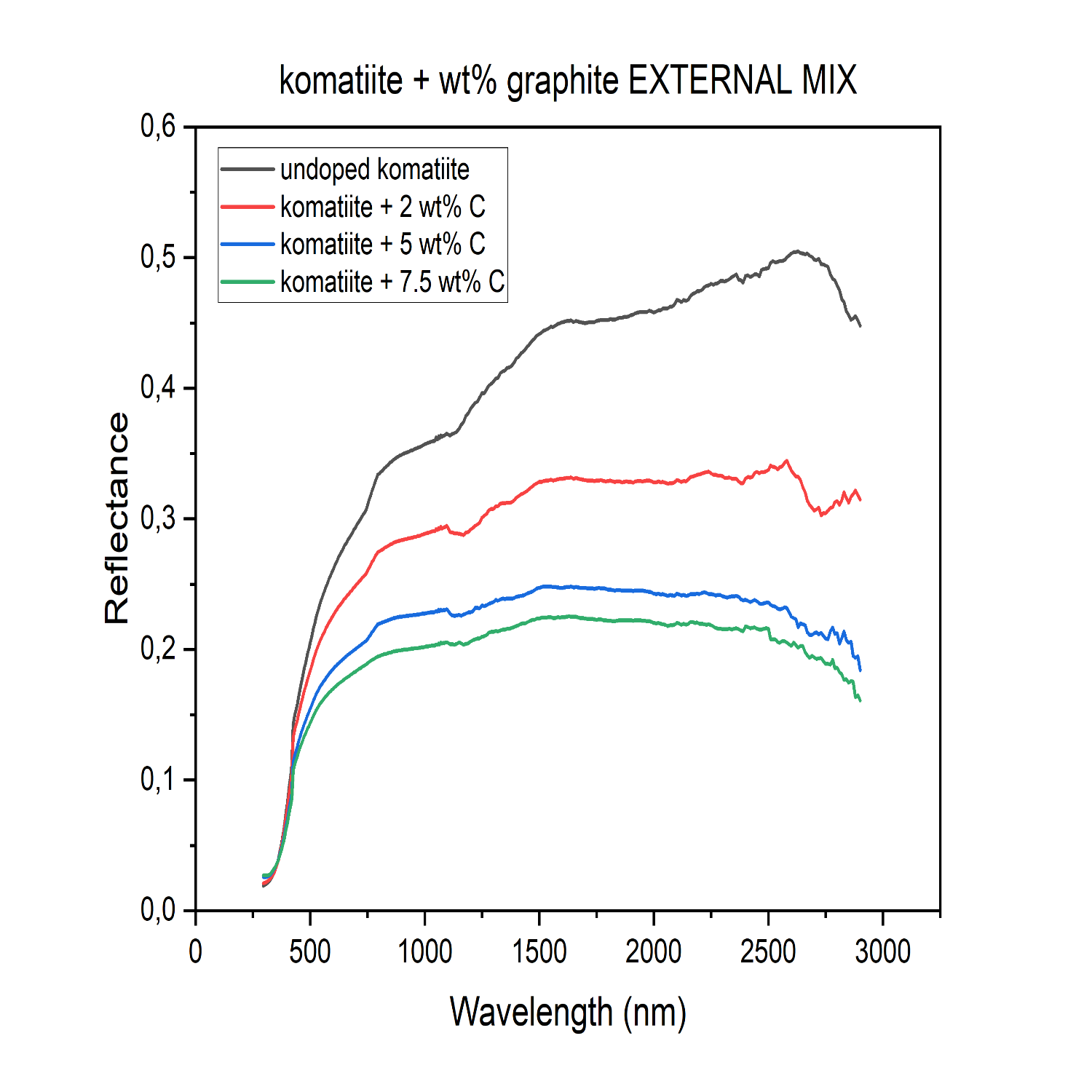
Figure 4: Spectral reflectance of the komatiite glasses with various wt% of graphite, designated as C, externally added. The spectra reveal that as the amount of graphite externally added to the glass increases, the reflectance decreases.
Caminiti, E., et al. (2024). Icarus, 420, 116191.
Lark, L. H., et al. (2023). Earth and Planetary Science Letters, 613, 118192.
Syal, M. B., et al. (2015). Nature Geoscience, 8(5), 352–356.
Weider, S. Z., et al. (2012). J. Geophys. Res., 117, E00L05.
Wurz, P., et al. (2025). The Planetary Science Journal, 6(1), 24.
How to cite: Incaminato, G., Vuori, M., Penttilä, A., Carli, C., Maturilli, A., Galiano, A., Petit, L., Ojha, N., Nasser, K., Vainio, M., and Muinonen, K.: Mercury surface UV-Vis-NIR spectral reflectance: Role of Graphite, EPSC-DPS Joint Meeting 2025, Helsinki, Finland, 7–12 Sep 2025, EPSC-DPS2025-1361, https://doi.org/10.5194/epsc-dps2025-1361, 2025.