- 1Institute for Planetary Materials, Okayama University, Misasa, 827 Yamada, Misasa, Tottori 682- 0193, Japan
- 2Space Park Leicester, University of Leicester, Leicester
Introduction
Data from NASA’s MESSENGER (MErcury Surface, Space ENvironment, GEochemistry, and Ranging) mission have revealed a chemically diverse surface on Mercury. Surface composition has been constrained using X-ray Spectrometry (XRS), Gamma-Ray Spectrometry (GRS), and Neutron Spectrometry (NS) [1], [2]. Despite regional variations, two common features stand out: a high sulfur concentration (up to 4 wt.%) and a notably low iron content (as low as 1 wt.%) [1], [2], [3], [4], [5], [6], [7]. These observations are consistent with extremely reducing conditions (oxygen fugacity, ƒO₂, between IW–3 and IW–7), which are markedly different from other terrestrial bodies. Under such reducing conditions, sulfur becomes lithophile and its solubility in FeO decreases [3]. As a result, the geochemical behaviour of major elements like Mg and Ca changes, with these typically lithophile elements partitioning into chalcophile phases [3], [4]. During Mercury’s magma ocean crystallization, sulfur would have progressively concentrated in the residual melt. The main predicted sulfides include Mg-rich niningerite (MgS) and Ca-rich oldhamite (CaS). Recent studies suggest that these sulfides, being less dense than the silicate matrix, may have floated or become sequestered in the upper mantle [3], [6]. However, experimental data on the formation of MgS and CaS, especially in Fe-free systems, are limited.
This study aims to create Mercury surface fully crystalline sample analogs that can be compared to results from remote sensing from the upcoming BepiColombo mission (e.g. using MIXS data). Controlled slow-cooling experiments in a piston-cylinder are used to ensure full crystallinity and simulate natural crystallization histories of each sample. Additionally, to understand the conditions required for the formation of various sulfide phases (e.g.FeS, CaS, MgS) under Mercury-like conditions, we try to change the samples with different conditions such as the presence of Fe, a range of sulfur contents (1–4 wt.%) and reducing conditions near and below the IW buffer.
Experimental Methods
Synthetic compositions were selected to represent the geochemical variability of Mercury’s surface, including 1) High-Mg Terrane (HMg), 2) Intermediate High-K Terrane (IHK), 3) North Low-Mg Terrane (N-LMg), and 4) Rachmaninoff Basin (RB),
[2], and regional divisions from [1], [7]. Synthetic analogs were prepared using a mixture of analytical-grade oxides and carbonates, which were decarbonated at 1000 °C for 4–10 hours. For samples containing Fe, reduction was carried out in a H₂–CO₂ gas mixing furnace to achieve oxygen fugacity (ƒO₂) below the iron-wüstite (IW) buffer following equilibrium: 2Fe(metal) + O2(gas) -> 2FeO(silicate). In case for Fe-free samples the samples were baked into 1000 °C. In case of more reduced compositions the addition of metallic Si was used in place of SiO₂ following equilibrium buffer: Si(metal) + O₂(gas) → SiO₂(silicate).
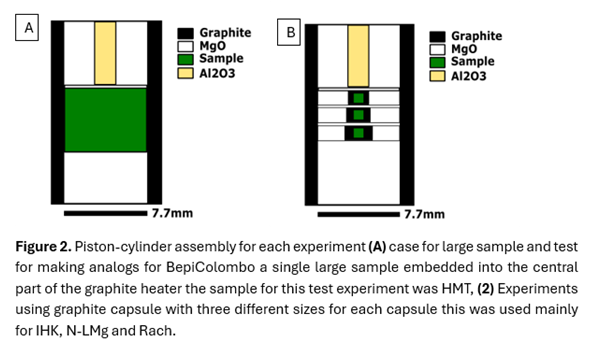
The powdered starting materials were loaded into graphite capsules (C-CO2 buffer) to maintain reducing conditions during the experiments. Capsules were placed in a piston-cylinder assembly (Fig. 2) and subjected to a pressure of 1 GPa. After reaching the pressure established samples were heated to above liquidus temperature and the slowly cooled to below solidus to promote crystallization. Post-run products were sectioned and analyzed using SEM/EDS.
Preliminary Results
HMg-Terrane:
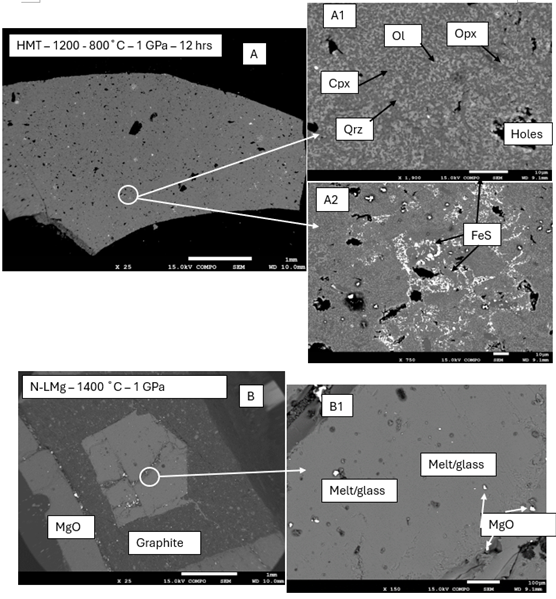
At 1 GPa, with slow cooling from 1200 °C to 800 °C over 12 hours, the sample yielded a multi-phase assemblage with <10% residual glass. Identified phases include quartz, orthopyroxene, clinopyroxene, olivine, and FeS (Fig. 1A).
N-LMg, IHK, RB Terranes:
The first experiment was done at 1 GPa and 1400 °C (rapid quenched), samples were fully glassy (Fig. 1B).
The second experiments on these three samples under slow cooling (1200 °C → 800 °C over 20 hours), samples developed multi-phase assemblages but retained >20% residual silicate glass. Dominant crystalline phases include pyroxene and quartz. Sulfur was retained in the melt, suggesting that S might have still been siderophile at fO2 buffer around -1IW.
Discussion
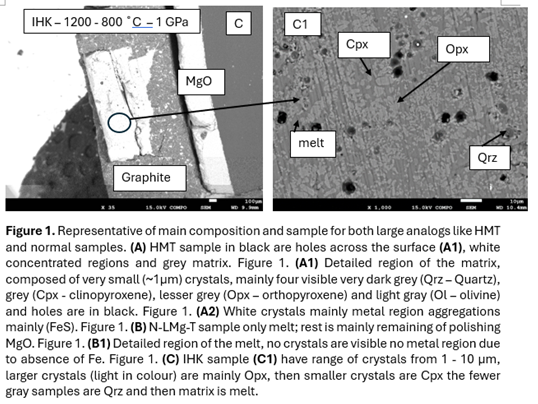
Initial experiments indicate that sulfur-bearing phases can crystallize under reducing conditions relevant to Mercury's upper mantle and crust with the presence of Fe. It is probable that at this stage the main factors are the siderophile nature of S and following this FeO(silicate) + S(metal) = FeS(metal) + O(gas). The evidence of such comes from High-Mg (HMg), produced a well-crystallized assemblage. Observed phase was FeS and small amount of S was present into the matrix (melts too small to detect) (Fig. 1A). Crystallisation of the remaining sample lead to the formation of Mg-rich olivine, orthopyroxene, then Ca-rich phase clinopyroxene and lastly quartz. The use of graphite capsules likely buffered the oxygen fugacity to values between IW and IW–1, this was confirmed by the presence of FeS and absence of FeO in HMg.
In contrast, quenched experiments of the North Low-Mg (N-LMg), Intermediate High-K (IHK), and Rachmaninoff Basin (Rach) analogs at 1400 °C were entirely glassy, indicating melt stability at temperature above liquidus. On the second set slow cooling (1200 °C to 800 °C over 20 hours), these samples produced partially crystallized assemblages still with >20% modal glass with Mg-rich orthopyroxene, clinopyroxene and quartz as dominant phases (see Fig. 1B). Despite reducing conditions near the IW buffer, neither CaS or MgS were not clearly observed, suggesting either (1) fO2 were not low enough to render S lithophile, also (2) insufficient sulfur concentration to start to form any solid solution, or (3) sulfur remained dissolved in the melt due to temperature not low enough to fully crystallise the samples.
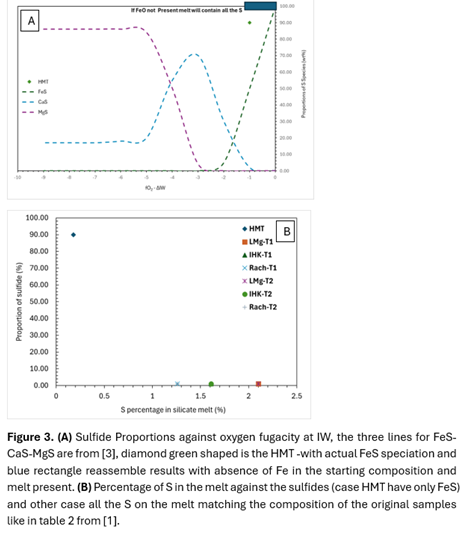
These observations imply that under IW to sub-IW conditions, sulfur retention in the melt is likely to persist until after crystallisation of the other major phases. Previous study [3] has shown that in this fO2 condition, FeS is the main to form and at lower fO2 CaS will start to form and then MgS (Fig. 3 A and B). However, the absence of Fe should be replaced with another form of sulfide in case of fully crystallinity.
Further work will explore the effect of increased sulfur content and variable Fe concentrations to better constrain sulfide saturation limits, the stability of CaS and MgS phases in Fe-free systems, and the implications for Mercury’s crustal sulfide distribution. These insights are crucial for interpreting remote sensing data from the BepiColombo mission, particularly the MIXS instrument suite.
Figures
How to cite: Diyalanthonige, D. H. F., Yoshino, T., Izawa, M. R. M., Martindale, A., and Barry, T. L.: Preliminary study on Mercury surface analogs evidence for Sulfur phases, EPSC-DPS Joint Meeting 2025, Helsinki, Finland, 7–12 Sep 2025, EPSC-DPS2025-1615, https://doi.org/10.5194/epsc-dps2025-1615, 2025.