EPSC Abstracts
Vol. 18, EPSC-DPS2025-2073, 2025, updated on 09 Jul 2025
https://doi.org/10.5194/epsc-dps2025-2073
EPSC-DPS Joint Meeting 2025
© Author(s) 2025. This work is distributed under the Creative Commons Attribution 4.0 License.
Structural Elucidation of N2O Clusters at Low Temperatures: A Matrix Isolation IR Spectroscopic and Computational study
- 1Indira Gandhi Centre for Atomic Research, a CI of Homi Bhabha National Institute (Mumbai), Kalpakkam, Tamil Nadu-603102, India
- 2Atomic and Molecular Physics Laboratory, Institute for Nuclear Research (Atomki), Debrecen 4026, Hungary
- 3Division of Geological and Planetary Sciences, California Institute of Technology, Pasadena, California, USA
Studies on the structures of nitrous oxide (N2O) and its clusters on Earth and in space are of interest primarily due to their astrochemical and atmospheric relevance. Being isoelectronic with CO2, N2O has a huge impact on atmospheric chemistry as it is one of the greenhouse gases that is found in the terrestrial troposphere[1] and, in the case of exoplanets, scientists consider N2O to be a potential remote biosignature for Earth-like planets, along with O2, O3, and CH4 [2,3].
The present study investigates the interactions that bind N2O molecules together within their aggregates at low temperatures using matrix-isolation infrared spectroscopy[4] coupled with insights from ab initio computations. The pnicogen character of the central nitrogen atom(s) is a major factor in determining the aggregation of N2O molecules and is substantiated by molecular topography that contributes to the stability. N2O is a very small molecule; it exists as a dimer even at room temperature favouring cluster formation at low temperature.
Spectral fingerprints in near-IR and mid-IR spectra are the key to the spectroscopic structural characterization of N2O and its aggregates [5,6]. A number of previous studies have reported the formation of N2O ices in various astrophysical environments[7], as it is considered to be an important tracer to quantify and characterize the abundance of N2 in extraterrestrial environments [8].
Furthermore, astrophysical N2O ice analogues have been bombarded by energetic electrons and ions to check the radiation stability of N2O ice by Almeida et al.[9], and very recently by Mifsud et al.[10], and the resultant dissociation products (NO2, NO and O3) have been characterized by FT-IR spectroscopy. Recently, N2 ice has been discovered by NASA in the atmosphere of Pluto[10], which can lead to the formation of N2O ice by reacting with an excited O atom, as suggested by Jamieson et al.[11]. A number of groups have studied the structure of the N2O molecule and its aggregation behaviour under matrix isolated conditions in solid N2[12], Ar[13], Xe[14], Ne[15] p-H2[16] matrices.
Previous studies have examined the structure of N2O clusters but have not quantified the forces holding them together, generally attributing them to van der Waals or dipole-dipole interactions. This study explores the possibility of pnicogen bonding due to the unique electronic structure of N2O. Dimers and trimers of N2O were synthesized at low temperatures in an argon matrix and analyzed using infrared spectroscopy and ab initio methods. The findings offer new insights into the nature of the interactions stabilizing these clusters, which will be discussed further at the meeting.
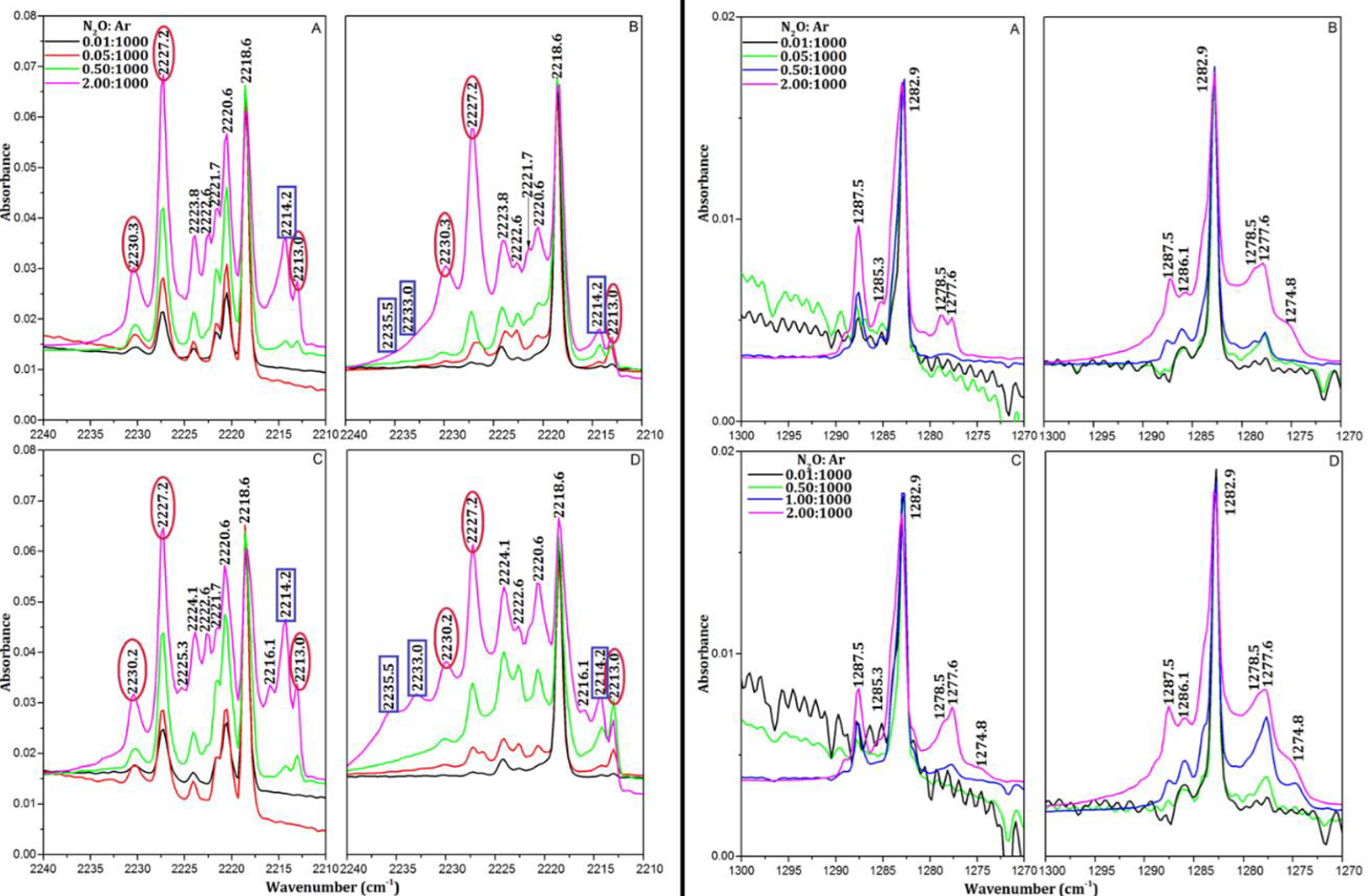
Fig. 1: Matrix isolation IR spectra of N=N stretching region (left) and of N-O stretching region (right) of N2O in Ar matrix using both effusive and supersonic molecular beam techniques.
References:
1. Sivaraman, B., Ptasinska, S., Jheeta, S. & Mason, N. J. Electron irradiation of solid nitrous oxide. Chem Phys Lett 460, 108–111 (2008).
2. Grenfell, J. L., Gebauer, S., V. Paris, P., Godolt, M. & Rauer, H. Sensitivity of biosignatures on Earth-like planets orbiting in the habitable zone of cool M-dwarf Stars to varying stellar UV radiation and surface biomass emissions. Planet Space Sci 98, 66–76 (2014).
3. Schwieterman, E. W. et al. Evaluating the Plausible Range of N2O Biosignatures on Exo-Earths: An Integrated Biogeochemical, Photochemical, and Spectral Modeling Approach . Astrophys J 937, 109 (2022).
4. Chandra, S., Suryaprasad, B., Ramanathan, N. & Sundararajan, K. Nitrogen as a pnicogen?: evidence for π-hole driven novel pnicogen bonding interactions in nitromethane–ammonia aggregates using matrix isolation infrared spectroscopy and ab initio computations. Physical Chemistry Chemical Physics 23, 6286–6297 (2021).
5. Sagan, C., Thompson, W. R., Carlson, R., Gurnett, D. & Hord, C. A search for life on Earth from the Galileo spacecraft. Nature 365, 715–721 (1993).
6. Gordon, I. E. et al. The HITRAN2020 molecular spectroscopic database. J Quant Spectrosc Radiat Transf 277, 107949 (2022).
7. Hudson, R. L. & Moore, M. H. The N 3 Radical as a Discriminator between Ion‐ irradiated And UV‐photolyzed Astronomical Ices . Astrophys J 568, 1095–1099 (2002).
8. Hudson, R. L. N 2 Chemistry in Interstellar and Planetary Ices: Radiation-driven Oxidation. Astrophys J 867, 160 (2018).
9. Almeida, G. C. et al. Processing of N2O ice by fast ions: Implications on nitrogen chemistry in cold astrophysical environments. Mon Not R Astron Soc 471, 1330– 1340 (2017).
10. Cruikshank, D. P. et al. The surface compositions of Pluto and Charon. Icarus 246, 82–92 (2015).
11. Jamieson, C. S., Bennett, C. J., Mebel, A. M. & Kaiser, R. I. Investigating the Mechanism for the Formation of Nitrous Oxide [N 2 O( X 1 Σ + )] in Extraterrestrial Ices . Astrophys J 624, 436–447 (2005).
12. Nxumalo, L. M. & Ford, T. A. IR spectra of the dimers of carbon dioxide and nitrous oxide in cryogenic matrices. J Mol Struct 327, 145–159 (1994).
13. Kudoh, S., Onoda, K., Takayanagi, M. & Nakata, M. N2O clusters in a supersonic jet studied by matrix-isolation infrared spectroscopy and density functional theory calculation. J Mol Struct 524, 61–68 (2000).
14. Lawrence, W. G. & Apkarian, V. A. Infrared studies in free standing crystals: N2O-doped Xe and Ar. J Chem Phys 97, 2224–2228 (1992).
15. Krueger, H. & Weitz, E. O(3P) atom lifetimes and mobilities in xenon matrices. J Chem Phys 96, 2846–2855 (1992).
16. Wan, L., Xu, G., Wu, L., Chen, Y. & Hu, S. M. Vibrational spectroscopy of N2O in solid neon matrices. J Mol Spectrosc 249, 65–67 (2008).
1. Sivaraman, B., Ptasinska, S., Jheeta, S. & Mason, N. J. Electron irradiation of solid nitrous oxide. Chem Phys Lett 460, 108–111 (2008).
2. Grenfell, J. L., Gebauer, S., V. Paris, P., Godolt, M. & Rauer, H. Sensitivity of biosignatures on Earth-like planets orbiting in the habitable zone of cool M-dwarf Stars to varying stellar UV radiation and surface biomass emissions. Planet Space Sci 98, 66–76 (2014).
3. Schwieterman, E. W. et al. Evaluating the Plausible Range of N2O Biosignatures on Exo-Earths: An Integrated Biogeochemical, Photochemical, and Spectral Modeling Approach . Astrophys J 937, 109 (2022).
4. Chandra, S., Suryaprasad, B., Ramanathan, N. & Sundararajan, K. Nitrogen as a pnicogen?: evidence for π-hole driven novel pnicogen bonding interactions in nitromethane–ammonia aggregates using matrix isolation infrared spectroscopy and ab initio computations. Physical Chemistry Chemical Physics 23, 6286–6297 (2021).
5. Sagan, C., Thompson, W. R., Carlson, R., Gurnett, D. & Hord, C. A search for life on Earth from the Galileo spacecraft. Nature 365, 715–721 (1993).
6. Gordon, I. E. et al. The HITRAN2020 molecular spectroscopic database. J Quant Spectrosc Radiat Transf 277, 107949 (2022).
7. Hudson, R. L. & Moore, M. H. The N 3 Radical as a Discriminator between Ion‐ irradiated And UV‐photolyzed Astronomical Ices . Astrophys J 568, 1095–1099 (2002).
8. Hudson, R. L. N 2 Chemistry in Interstellar and Planetary Ices: Radiation-driven Oxidation. Astrophys J 867, 160 (2018).
9. Almeida, G. C. et al. Processing of N2O ice by fast ions: Implications on nitrogen chemistry in cold astrophysical environments. Mon Not R Astron Soc 471, 1330– 1340 (2017).
10. Cruikshank, D. P. et al. The surface compositions of Pluto and Charon. Icarus 246, 82–92 (2015).
11. Jamieson, C. S., Bennett, C. J., Mebel, A. M. & Kaiser, R. I. Investigating the Mechanism for the Formation of Nitrous Oxide [N 2 O( X 1 Σ + )] in Extraterrestrial Ices . Astrophys J 624, 436–447 (2005).
12. Nxumalo, L. M. & Ford, T. A. IR spectra of the dimers of carbon dioxide and nitrous oxide in cryogenic matrices. J Mol Struct 327, 145–159 (1994).
13. Kudoh, S., Onoda, K., Takayanagi, M. & Nakata, M. N2O clusters in a supersonic jet studied by matrix-isolation infrared spectroscopy and density functional theory calculation. J Mol Struct 524, 61–68 (2000).
14. Lawrence, W. G. & Apkarian, V. A. Infrared studies in free standing crystals: N2O-doped Xe and Ar. J Chem Phys 97, 2224–2228 (1992).
15. Krueger, H. & Weitz, E. O(3P) atom lifetimes and mobilities in xenon matrices. J Chem Phys 96, 2846–2855 (1992).
16. Wan, L., Xu, G., Wu, L., Chen, Y. & Hu, S. M. Vibrational spectroscopy of N2O in solid neon matrices. J Mol Spectrosc 249, 65–67 (2008).
How to cite: Mahapatra, N., Chandra, S., Ramanathan, M. N., and Sundararajan, M. K.: Structural Elucidation of N2O Clusters at Low Temperatures: A Matrix Isolation IR Spectroscopic and Computational study, EPSC-DPS Joint Meeting 2025, Helsinki, Finland, 7–12 Sep 2025, EPSC-DPS2025-2073, https://doi.org/10.5194/epsc-dps2025-2073, 2025.