- 1University of Arkansas, Fayetteville AR USA
- 2German Aerospace Center (DLR), Institute of Space Research, Berlin, Germany
- 3European Space Agency ESA, ESTEC, Noordwijk, The Netherlands
Introduction: We are investigating the ability for the BepiColombo mission to identify sulfides on the surface of Mercury with the MIXS x-ray spectrometer and the MERTIS thermal infrared spectrometer. The phase in which sulfur exists on Mercury’s surface is unknown; the Mercury Radiometer and Thermal Infrared Spectrometer (MERTIS) will be able to examine the mineralogical phases that exist on the surface [1,2]. This paired with the Mercury Imaging X-ray Spectrometer’s (MIXS) ability to determine the elemental composition of the first 10-20 μm of the surface will provide a detailed map of the composition [3].
Spectra were collected at the Deutsches Zentrum für Luft- und Raumfahrt in the Planetary Spectroscopy Laboratory (PSL) for this project [4]. Three sulfides, CaS, MgS, and FeS were the primary targets of this study, and mixtures of the endmember elements were created to compare with the sulfides. Emissivity and reflectance measurements were taken at the same wavelengths as those covered by the MERTIS instrument to determine if sulfides will be detectable by BepiColombo. Our aim is to observe any spectral features that can be attributed to the sulfide bonds.
Methods: A set of 10 samples was measured in reflectance and emissivity. The three sulfides, CaS, MgS, and FeS, and corresponding mixtures were created of their two endmembers at the same weight percent as those found in the sulfides. For instance, our Ca+S mixture was 55.6% Ca and 44.4% S to simulate the weight percent of CaS. Measurements of the individual elements were also taken.
Emissivity measurements were taken with a Bruker Vertex 80V FTIR attached to a vacuum chamber with an internal heating mechanism [4]. Samples were placed in steel cups that would be heated through an induction system from below. Measurements were taken in MIR wavelengths (2-16 microns) to to cover the spectral range of the the MERTIS instrument (7-14 microns) [1]. Measurements were taken at 250, 300, 350, 400, and 450°C. The procedure for sulfur bearing samples was altered to allow for proper signal collection after sulfur outgassing. A beaker was placed on top of the sample, and it was heated to 250°C to allow sulfur to offgass and adhere to the beaker rather than the chamber ,mirror, and instrument itself. The sample was cooled, the beaker was then removed and the data collection continued as normal.
Bidirectional reflectance measurements were taken of each sample before and after the heating process. These were taken across multiple wavelengths including UV, VIS, VNIR and MIR. This allowed us to spot the irreversible spectral changes to the samples due to the heating process.
Results: Calcium and magnesium sulfides were both stable across the simulated Mercury temperatures and their distinct absorption features could be observed in each measured temperature. The positions of the spectral feature changed due to the increase in temperature, but important diagnostic features remained detectable, as seen in figure 1.
Some features in the sulfide spectra were not observed in those of the sulfur mixtures, displaying the ability for the sulfides to be distinguishable from mixtures. An example can be seen in figure 2 where the CaS spectrum has a large emission feature at 8-9 microns that is not seen in either the Ca or Ca+S spectra. An explanation of this distinction is that elemental sulfur found in the mixtures is not stable and degasses at high temperatures, which would explain the similarity of the mixture and the Ca spectra at high temperatures. It is commonly understood that sulfur is unstable at high temperatures, but our experiments also show that the sulfur remains in the system if bound to other molecules in a sulfide. This is demonstrated in both the CaS and MgS samples.
Iron sulfide proved to be harder to distinguish from iron sulfur mixtures due to the high emission rates of both. The spectra were bright and had few features. We found that at the wavelengths used by the MERTIS instrument, CaS and Mgs should be able to be identified but FeS cannot.
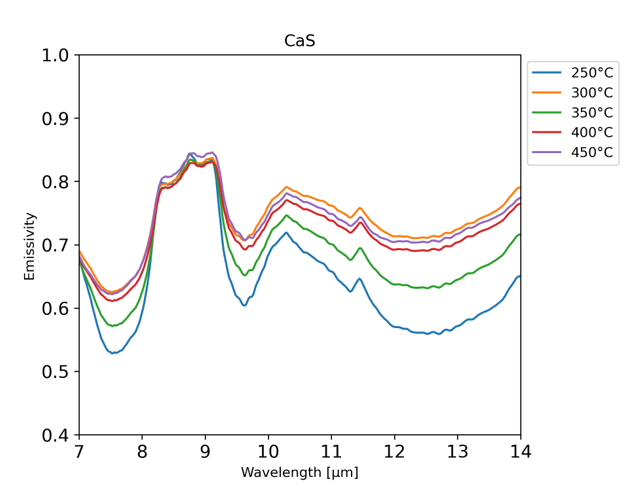
Figure 1: Calcium Sulfide spectra at each measured temperature, 250°C to 450°C
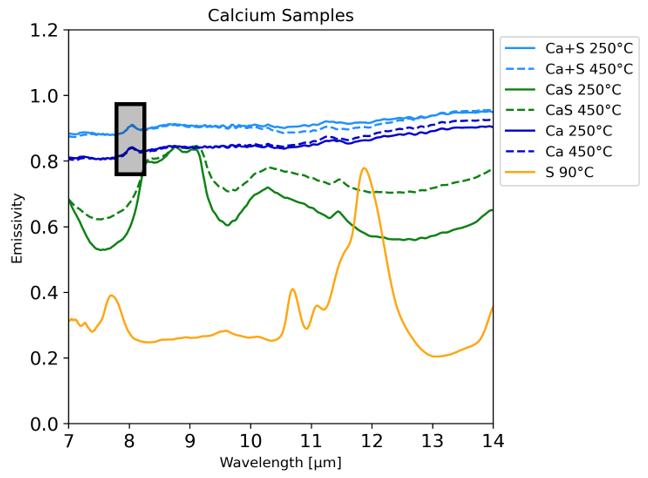
Figure 2: Highest (450°C) and lowest (250°C) temperature spectra for all calcium samples, Ca+S mixture, CaS, and Ca. The sulfur spectra included was collected at 90°C due to the instability of pure sulfur.
Significance of this Work: Bepicolombo’s MERTIS and MIXS instruments will soon provide new data that will aid in our understanding of Mercury’s surface composition. It is currently unknown the state in which the sulfur on the surface exists. MIXS will aid in identifying the sulfur and other elements on the surface, and MERTIS will provide mineralogical phase data on these areas. Laboratory measurements are necesary to understand this future data and have a dataset with which to compare. Our work shows the spectral features which will be observed by MERTIS if sulfides or sulfur mixtures are observed.
Acknowledgements: The University of Arkansas’ Sturgis International Fellowship allowed the first author to visit the DLR in Berlin for four months to collect this data.
References : [1] Hiesinger et al. (2020) Studying the Composition and Mineralogy of the Hermean Surface with the Mercury Radiometer and Thermal Infrared Spectrometer (MERTIS) for the BepiColombo Mission: An Update, Space Sci Rev, 216, no. 6, p. 110. [2] Sprague et al. (1995) Sulfur at Mercury, Elemental at the Poles and Sulfides in the Regolith, Icarus 118 p.211-215. [3] Bunce et al. (2020) The BepiColombo Mercury Imaging X-Ray Spectrometer: Science Goals, Instrument Performance and Operations, Space Sci Rev, 216, no. 8, p. 126. [4] Maturilli et al. (2018) The Planetary Spectroscopy Laboratory (PSL) – wide spectral range, wider sample temperature rage; Proc. SPIE 10765, Infrared Remote Sensing and Instrumentation XXVI, 107650A.
How to cite: Merchak, E., Maturilli, A., Alemanno, G., and Helbert, J.: Laboratory investigation of spectral signatures of Sulfides and Sulfur Mixtures as seen by the MERTIS and MIXS Instruments, EPSC-DPS Joint Meeting 2025, Helsinki, Finland, 7–12 Sep 2025, EPSC-DPS2025-950, https://doi.org/10.5194/epsc-dps2025-950, 2025.