Introduction:
The LEOrigin payload is an initiative led by young professionals at the European Space Agency. LEOrigin is a pioneering astrobiology experiment investigating how space radiations may trigger the formation of life’s building blocks. The payload is designed for launch in mid-2027 aboard Space Rider, which will remain in Low Earth Orbit (LEO) for two months and features an exposure facility.
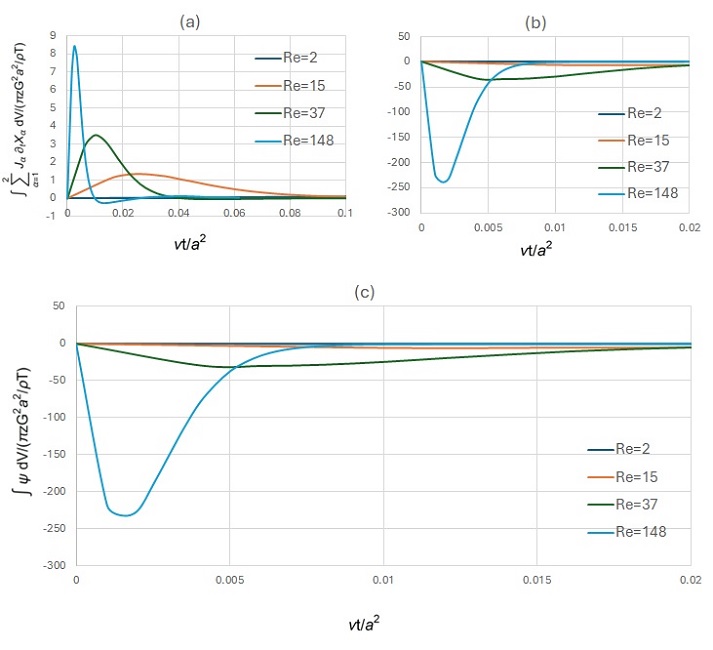
The experiment focuses on the conversion of formamide [1] – a simple, one-carbon molecule found throughout the Universe (interstellar medium, comets, and protoplanetary disks) – triggered by energetic UV and cosmic radiations catalyzed by asteroid-, meteorite- and comet-like minerals, in space environment conditions, see (Fig. 1).
Inspired by the groundbreaking Miller-Urey experiment [2], which demonstrates the synthesis of organic compounds under early Earth-like conditions, and the discovery that silica played a major role in these reactions [3], LEOrigin builds on these findings by testing how formamide condensation catalyzed by silicate minerals common in asteroids and comets, can lead to the formation of nucleobases and other life-relevant compounds.
Recent discoveries highlight the importance of studying these processes: the OSIRIS-REx mission detected amino acids (including 14 of the 20 used in terrestrial biology) and all five RNA/DNA nucleobases on asteroid Bennu [4], while the Hayabusa-2 mission found the uracil nucleobase on asteroid Ryugu [5]. These findings suggest that life’s precursors may have formed on multiple bodies in the early solar system (including Earth) and that asteroids could have also delivered additional complex molecules to our planet.

Formamide: Reactive to UV Light and Cosmic Radiations.
Previous research has demonstrated that formamide condensation, when catalyzed by silica-rich [1] or photocatalytic semiconductive minerals [6], can produce a wide panel of organic molecules crucial for genetic and metabolic processes. Condensation occurs when the reaction’s activation energy is reached, either through thermal heating or exposure to high-energy irradiation (protons, UV).
This has been confirmed through various laboratory experiments, where UV-lamps [6] and proton irradiation [7] were used to mimic space-like conditions, providing valuable insights into the prebiotic chemistry of formamide. LEOrigin will, for the first time, recreate these experiments in orbit, utilizing the high energy radiation in space.
Choice of Catalytic Mineral Samples:
LEOrigin will carry a set of four minerals, selected to be representative of asteroids, comets, and Martian soil & meteorites, as well as to replicate ground-based experiments:
- Titanium dioxide (TiO2): a photocatalytic semiconductive mineral, selected based on the successful ground-based UV exposure experiment by Senanayake et al. [6].
- Olivine: a silicate mineral abundant in carbonaceous asteroids and the dominant mineral in most chondritic meteorites [8]. Crystalline and amorphous olivine was also observed in the dust around comet 9B-Tempel 1 [9].
- Smectite (Saponite/Nontronite): Fe/Mg-smectite is the most abundant phyllosilicate identified on the surface of Mars [10] almost always found in ancient rocks ~4 Gy old, suggesting that this mineral may have formed early in the life of the Solar System. Smectites were also detected in achondrite Martian meteorites (e.g., saponite in Nakhla [11]), they are present in some chondrite meteorites (e.g., smectites in Ivuna and Orgueil were found intimately mixed with a carbon-rich matrix attributed to pre-terrestrial acqueous alteration [12]), and is also the most common clay mineral in comets when detected (e.g., nontronite in comet 9B-Tempel 1 [13]).
- Vermiculite (Oxia Planum analog): Vermiculite is a Fe2+/Mg-rich phyllosilicate, which constitutes the main spectral signature found at the ~ 4 Gy old clay-bearing Oxia Planum landing site for the ExoMars Rover mission [14]. Assessing the role of this mineral as a catalyst in the formation of prebiotic molecules would thus create synergies and contribute to the objectives of the ESA astrobiology rover mission.
Payload Design and Configuration:
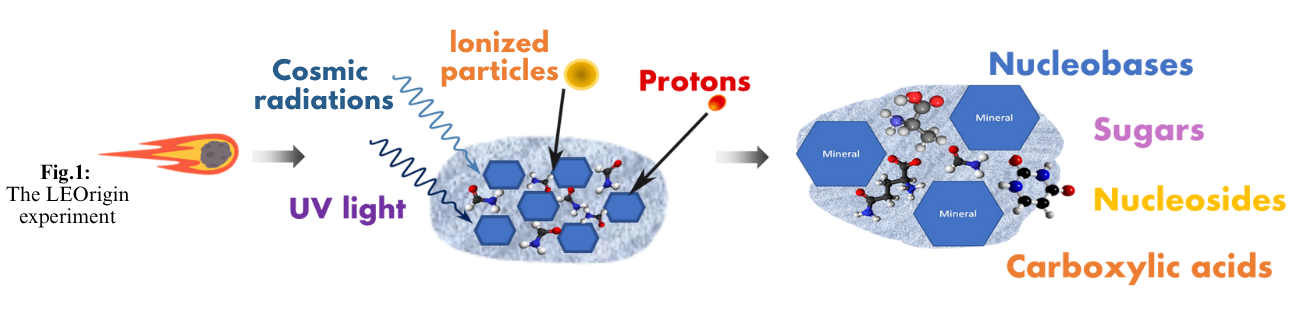
The payload is composed of 42 cells (Fig. 2 A&B) divided into two superimposed stacks. Each stack consists of 14 pelletized minerals injected with formamide or formamide+water; 4 control cells containing only formamide or formamide+water; and 3 cell spots for UV and thermal sensors. The top stack will be exposed to UV light while the second will serve as dark control. The cell windows are made from MgF2, which is transparent to UV light and the cell housing is chosen such that no interference with the formamide condensation is expected (Fig. 2C). Top cells will be exposed to solar and cosmic radiations when Space Rider Cargo Bay doors open.

References: [1] Saladino, R. et al. Phys Life Rev 9, 84–104 (2012). [2] Miller, S. L. & Urey, H. C. Science (1979) 130, 245–251 (1959). [3] Criado-Reyes, J. et al. Sci Rep 11, 21009 (2021). [4] McCoy, T. J. et al. Nature 637, 1072–1077 (2025). [5] Oba, Y. et al. Nat Commun 14, 1292 (2023). [6] Senanayake, S. D. et al. Proceedings of the National Academy of Sciences 103, 1194–1198 (2006). [7] Saladino, R. et al. Proceedings of the National Academy of Sciences 112, (2015). [8] Sunshine, J. M. et al. Meteorit Planet Sci 42, 155–170 (2007). [9] Kelley, M. S. & Wooden, D. H. Planet Space Sci 57, 1133–1145 (2009). [10] Carter, J. et al. J Geophys Res Planets 118, 831–858 (2013). [11] Hicks, L. J. et al. Geochim Cosmochim Acta 136, 194–210 (2014). [12] Morlok, A. et al. Geochim Cosmochim Acta 70, 5371–5394 (2006). [13] Lisse, C. M. et al. Science (1979) 313, 635–640 (2006). [14] Quantin-Nataf, C. et al. Astrobiology 21, 345–366 (2021).