Excitation and ionization processes of Pyridine molecule induced by low-energy electron impact
- Comenius University, Faculty of Mathematics, Physics and Informatics, Department of experimental physics, Slovakia (janblasko871@gmail.com)
Introduction
The studied molecule belongs to cyclic hydrocarbons with one nitrogen atom. The molecule is found, for example, in nicotine and is also found in B vitamins, which are important for cell metabolism [1]. Pyridine is also used as a solvent or precursor for agrochemicals in fertilizers [2]. Pyridine attracts attention not only due to its industrial and biological consequences, but also due to astrochemistry. B vitamins have also been found on the surface of meteorites [3]. Pyridine was for example identified in the methanol extract of the Murchison meteorite, which is probably formed by the reaction of NH3 and aldehydes. The identification was done by liquid chromatography and mass spectrometry. This is one of the reasons why a thorough knowledge of the basic physical and chemical properties of this molecule is important.
Experiment
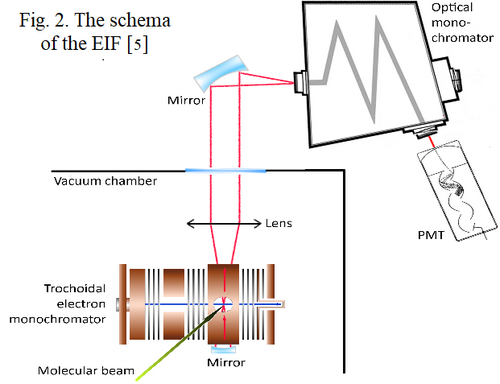
For investigation of electron induced processes of molecules an experiment with crossed electron and molecular beam was used. The sample (C5H5N, CAS: 110-86-1, purity: 99.8 %, Sigma Aldrich) is in liquid state in laboratory conditions and its vapors were introduced into the reaction chamber through molecular beam source (MBS) using capillary to generate effusive beam. The electrons were produced by trochoidal electron monochromator (TEM) generating an electron beam with a well specified energy with an energy resolution 300 meV FWHM. The products of these reactions were analyzed by Crossed Electron Molecular Beams Ionization Apparatus (CEMBIA) [4]. and Electron Induced Fluorescence Apparatus (EIF) [5].
In the case of the CEMBIA experiment, the ions generated by the electron-molecular collision are directed by an electric field to a quadrupole mass spectrometer (QMS), where they are separated based on their mass to charge ratio. Ions that pass through the QMS are detected by the channeltron (CEM). In the case of the EIF experiment, the photons emitted during the deexcitation of the excited reaction products pass through the optical system from the vacuum chamber to the optical monochromator and are detected by a photomultiplier operating in the photon counting mode.
Results
Using CEMBIA apparatus we have observed electron ionization and dissociative electron ionization of pyridine by electron impact. In the Fig. 3. the mass spectrum of the pyridine molecule measured at an electron energy of 70 eV is shown. The parent positive ion, denoted P+, weighs 79 amu. At 80 amu there is the isotope of the molecule. Subsequently, we see the dissociation of pyridine into fragments. Firstly, the hydrogen is sequentially dissociated from the parent. Subsequently, a very nice sequential carbon dissociation is seen, starting from a mass at 74 amu with the C5N+ product. Subsequently, additional carbon is likely to dissociate to the C4N+ product at 62 amu. Sequential dissociation probably continues through masses and products at 50 amu C3N+, 38 amu C2N+, 26 amu CN+ up to a mass at 14 amu, where we detected a fragment of the N+ nitrogen atom. Similar dissociations are intertwined across the spectrum. We were mainly interested in two fragments, C+ (mass 12) and CH+ (mass 13). The reason is the fluorescence spectrum measured by EIF apparatus in which we see the transitions associated with the positive ions C+ and CH+. The fluorescence emission spectrum of the pyridine molecule induced by monochromatic electrons is shown in Fig 4. In the spectrum we can see CH+ at the wavelength of 351 nm and 423 nm and C+ at the wavelength of 391.75 nm. Other detected products are not ionized. The fluorescence spectrum is measured at an interacting electron energy of 70 eV. The measurement range of the fluorescence spectrum is from 200 to 700 nm, with no signal being detected from 200 to 310 nm.
In the range from 525 to 700 nm, we detected only hydrogen Balmer series Hα line. The hydrogen Balmer series is also observed and identified. In Fig 5. we show an illustrative example of a 2D spectral map of the fluorescence cross section. The 2D map is made by measuring spectra in the range from 200 to 700 nm in steps of 0.1 nm for a constant energy of interacting electrons per measurement. The measurements are repeated in 5 eV steps ranging from 15 eV up to 100 eV.
Acknowledgments
This work was supported by projects APVV-19-0386 and VEGA 1/0489/21. This project has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No 692335.
References
[1] I. Linert, M. Zubek, Eur. Phys. J. D (2016) 70: 74
[2] M. A. Smialek and col., Eur. Phys. J. D (2016) 70: 42
[3] Y. Yamashita, H. Naraoka, Geochemical J. (2014) 48, 519-525
[4] M. Lacko, P. Papp and Š. Matejčík, J. Chem. Phys. (2018) 148, 214305.
[5] M. Danko and col., Plasma Sources Sci Technol (2016) 25, 065007
How to cite: Blaško, J., Országh, J., Stachova, B., Papp, P., and Matejčík, Š.: Excitation and ionization processes of Pyridine molecule induced by low-energy electron impact, European Planetary Science Congress 2021, online, 13–24 Sep 2021, EPSC2021-28, https://doi.org/10.5194/epsc2021-28, 2021.

