SB3
Dear SB3 contributers,
Thank you again for your contributions during the live session on Thursday, September 16.
In the middle of the conference, I would like to ask you to please answer any questions you may have via Slack in order to promote the exchange of results.
So check back from time to time.
Gabriele
Session assets
Please decide on your access
Please use the buttons below to download the presentation materials or to visit the external website where the presentation is linked. Regarding the external link, please note that Copernicus Meetings cannot accept any liability for the content and the website you will visit.
Forward to presentation link
You are going to open an external link to the presentation as indicated by the authors. Copernicus Meetings cannot accept any liability for the content and the website you will visit.
We are sorry, but presentations are only available for users who registered for the conference. Thank you.
Oral and Poster presentations and abstracts
Ceres, dwarf planet of the main asteroid belt, is considered a relic ocean world since the Dawn mission discovered evidences of aqueous alteration and cryovolcanic activity [1]. Unexpectedly, a variety of ammonium-rich minerals were identified on its surface, including phyllosilicates, carbonates, and chlorides [2]. Although from the Dawn’s VIR spectroscopic data it was not possible to specify the exact type of phyllosilicates observed, montmorillonite is considered a good candidate owing to its ability to incorporate NH4+ in its interlayers [3]. Ammonium-rich phases are usually found at greater distances from the Sun. Hence, the study on their stability at environmental conditions relevant to Ceres’ interior and of its regolith can help elucidate certain ambiguities concerning the provenance of its precursor materials.
In this study, it was investigated the changes in the spectroscopic signatures of the clay mineral montmorillonite after (a) being immersed in ammonium chloride aqueous solution and, subsequently, (b) washed with deionized water. After each treatment, samples were submitted to different environmental conditions relevant to the surface of Ceres. For one experiment, they were frozen overnight at 193 K, and then subjected to 10-5 bar for up to 4 days in a Telstar Cryodos lyophilizer. For the other, they were placed inside the Planetary Atmospheres and Surfaces Chamber (PASC) [4] for 1 day at 100 K and 5.10-8 bar. The combination of different techniques, i.e., Raman and IR spectroscopies, XRD, and SEM/EDX, assisted the assignment of the bands to each particular molecule. In this regard, the signatures of the mineral external surface were distinguished from the interlayered NH4+ cations. The degree of compaction of the samples resulted crucial on their stability and spectroscopic response, being stiff smectites more resistant to low temperatures and vacuum conditions. In ground clay minerals, a decrease in the basal space with a redshift of the interlayered NH4+ IR band was measured after just 1 day of being exposed to vacuum conditions.
Acknowledgments
This work was supported by the Spanish MINECO projects ESP2017-89053-C2-1-P and PID2019-107442RB-C32, and the AEI project MDM‐2017‐0737 Unidad de Excelencia “María de Maeztu”.
References
[1] De Sanctis et al., Space Sci. Rev. 216, 60, 2020
[2] Raponi et al., Icarus 320, 83, 2019
[3] Borden and Giese, Clays Clay Miner. 49, 444, 2001
[4] Mateo-Marti et al., Life 9, 72, 2019
How to cite: Munoz-Iglesias, V., Fernández-Sampedro, M., Gil-Lozano, C., J. Bonales, L., Ercilla Herrero, O., Valles González, M. P., Mateo-Martí, E., and Prieto-Ballesteros, O.: Characterization of NH4-montmorillonite coexisting with NH4Cl salt at different aggregation states. Application to Ceres., Europlanet Science Congress 2021, online, 13–24 Sep 2021, EPSC2021-11, https://doi.org/10.5194/epsc2021-11, 2021.
Introduction
The VIR spectrometer on board the NASA’s Dawn spacecraft allowed providing important clues the mineralogical composition of the Ceres regolith (De Sanctis et al., 2015) and of the bright areas widespread across its surface (Palomba et al., 2019; Carrozzo et al., 2018). Some bright spots are thought to be the result of phenomena like cryovolcanism (Ruesch et al., 2016; Russell et al., 2016) and post-impact hydrothermal activities (Bowling et al., 2016). The study of Ceres bright areas is important to understand in more detail the mineralogical composition of the subsurface materials that could host water ice (Prettyman et al., 2017; Schmidt et al., 2017) or have been under aqueous alteration (De Sanctis et al., 2016).
In this study different bright areas of Haulani crater (e.g. Southern floor, i.e. ROI3 and North-east crater wall, i.e. ROI4) on Ceres have been studied by creating different analogue mixtures and comparing them with Dawn VIR data. The end-members have been identified based on previous studies (Tosi et al. 2018, 2019) and the analogue mixtures have been produced with grain size 50-100 µm for two bright crater regions. The spectra of two initial analogue mixtures have been acquired in the VIS-NIR spectral range (0.35-4.5 µm) at low temperature, i.e. from 200 K to 300 K similar to Haulani by using the Cold Spectroscopy Facility (CSS; https://cold-spectro.sshade.eu) (IPAG, France).
Scientific goals and method
The main scientific objectives of this study are: 1) the study of two different bright areas of Haulani crater (hereafter called ROI3 and ROI4, Figure 1) on Ceres in order to study the mineralogy of the sub-surface materials starting from results inferred by Dawn VIR; 2) the identification of the end-members of mineral mixtures of bright areas and production of endmembers and analogue mixtures with grain size 50-100 µm; 3) the acquisition of reflectance spectra of end-members and analogue mixtures in the VIS-NIR spectral range (0.35-4.5 µm); 4) the analysis of appropriate spectral parameters of reflectance spectra and comparison with those obtained by VIR data.
In particular, spectral parameters of mixtures will be estimated, focusing on Band Center (BC), Band Depth (BD), Full Width Half at Maximum (FWHM) of bands, reflectance level and spectral slopes (estimated between 1.2 and 1.9 µm). The spectral parameters of analogue mixtures have been compared with the VIR data corresponding to the selected area in order to constrain their mineralogical composition.
Data analysis and conclusion
Two analogue mixtures (50-100 µm), here called A3-1 and A3-2 have been produced by using the end-members Antigorite (Mg-phyllosilicate); NH4-montmorillonite (ammoniated phyllosilicate); anhydrous Sodium Carbonate (Na-carbonate); Graphite (dark component), Illite (Phyllosilicates) to simulate the two bright crater regions (Figure 1, i.e. southern floor and red spot or ROI3, i.e. north-east inner crater wall or ROI4). Reflectance spectra of the two mixtures have been acquired in the VIS-NIR spectral range (0.35-4.5 µm) at cold temperature, i.e., from 200 K to 300 K (phase angle of 30°) with the SHINE spectro-gonio-radiometer equipped with the CARBONIR simulation chamber (sample in inner cell filled with few mbar of pure N2 gas) at the Cold Spectroscopy Facility (CSS) in IPAG, France (Figure 1, Right). Finally, the analysis of spectral parameters of the reflectance spectra (mainly relative to the absorption bands at 2.7, 3.1, 3.4 µm) and the comparison with VIR data have been performed. The acquired spectra have been finally converted in radiance factor.
A first analysis shows that the Mixture A3-1 and A3-2 are not well representative due to the high amount of dark components (up to 86 % for A3-1) and missing Na-carbonate bands (for A3-2). Thus, the A3-2 has been modified (by producing the intermediate mixture) and by reaching 9 % for Na-carbonate, 32 % of dark component (i.e. carbon black) and 25 % of NH4-Montmorillonite in the final mixture named as A3-8. Finally, graphite and NH4-montmorillonite have been added to the A3-8 mixture, obtaining the last mixture A3-9. Thanks to carbon black the reflectance level compared with Haulani spectra is more similar. The analysed mixture were heated in the furnace in air at 120°C for 2 hours before each measurement and then placed in the sample holder under vacuum to remove the adsorbed H2O.
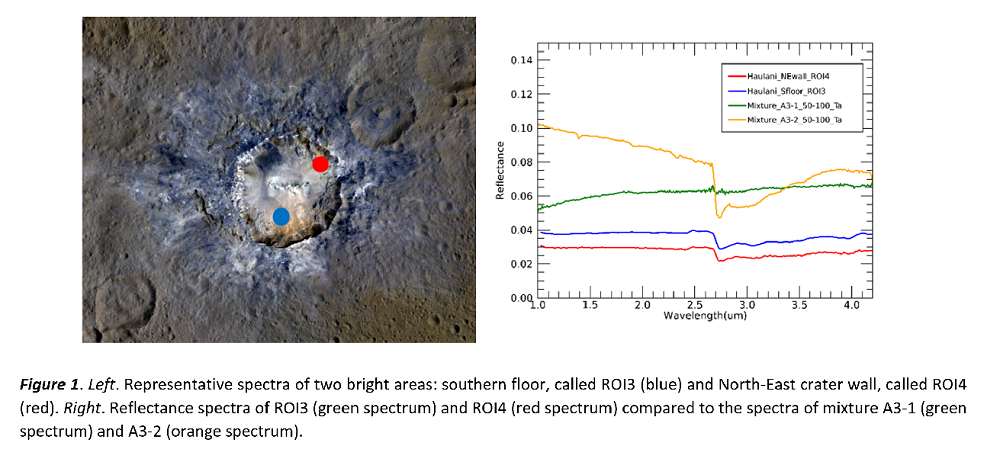
The mixtures with a reflectance spectrum similar to the spectra of ROI3 and ROI4 have been analysed in detail. By the spectral analysis, the Mixture A3-8 shows the most representative reflectance spectrum for the Haulani’s areas of interest (even if the difference in the reflectance level is probably due to opaque end-member composition) and exhibits BD values for the 2.7, 3.1 and 3.4 µm bands that are the closest one to the ROI3 and ROI4. The width of the 3.1 µm band (3.1FWHM) of A3-8 has a value similar to the ROI4 (about 0.15). In particular, the 2.7 BD is about 13% lower than ROI3 and ROI4, the 3.1BD is 5-9% higher while the 3.4BD has the same value of ROI4 and 11% lower than ROI3. A more in-depth analysis of the data is in progress.
Besides, in order to better reproduce Haulani areas some improvements may be performed, e.g., by adding a low amount of hydrous natrite , e.g. 2-8%, to assess the role of this component found in Haulani bright areas and how it contributes to the 2.7 µm spectral band.
References
Carrozzo, F.G., et al., 2018. Nature, Sci. Adv. 4 (3); De Sanctis. M. C. et al., 2015. Nature 528, pp. 241-244; De Sanctis, M.C., et al., 2016. Nature 536. Issue 7614, 54–57; Palomba, E., et al., 2019. Icarus 320, 202–212; Prettyman T. H., et al., 2017. Science 355:55–59; Ruesch, O., et al., 2016. Science 353 (6303); Russell, C.T., et al., 2016. Science 353 (6303), 1008–1010; Schmidt B. E. et al., 2017. Nature Geoscience 10:338–343; Tosi, F. et al., 2018. M&P Sci. 53, Nr.9, pp. 1902-1924; Tosi, F. et al., 2019. Icarus 318, pp.170-187.
How to cite: Dirri, F., Galiano, A., Longobardo, A., Palomba, E., Schmitt, B., Beck, P., Poch, O., and Brissaud, O.: VIS-NIR reflectance analysis of analogue mixtures representative of Haulani crater on Ceres to assess the mineralogical composition of bright areas, Europlanet Science Congress 2021, online, 13–24 Sep 2021, EPSC2021-397, https://doi.org/10.5194/epsc2021-397, 2021.
Summary
Ceres is the largest object in the Solar System main belt. Clearly, Ceres experienced extensive water-related processes and geochemical differentiation and nowadays it is a body with a complex geological and chemical history [1]. Its surface is characterized by dark materials, phyllosilicates, ammonium-bearing minerals, carbonates, water ice, and salts. In addition to a global presence of carbon bearing chemistry, local concentration of aliphatic organics has been detected by Dawn [2].
In this context, we have started a series of laboratory spectroscopy measurements targeted to study the physicochemical interactions between organic material and minerals possibly present on Ceres. The goal is to understand the transformations induced on these samples by ultraviolet radiation, neutral atoms, and fast ions, under experimental conditions that simulate the environment of Ceres. The spectroscopic data obtained in laboratory experiments allow, through the comparison with the observations of the VIR spectrometer aboard the Dawn mission, to clarify the nature and origin of organic material identified on Ceres.
Introduction
Organic material in the minor bodies of the Solar System is an important component to understand planetary evolution and, eventually, the origin of life. Nevertheless, our knowledge on the subject is still limited. Recently the Dawn mission, thanks to the data collected by the Italian instrument VIR [3], showed clear evidence of a high amount of aliphatic organic material on the surface of Ceres [4, 5, 6] (Fig 1). This evidence has raised new questions about the origin and preservation of this material, especially when considering its high estimated abundance and the mineralogical context.
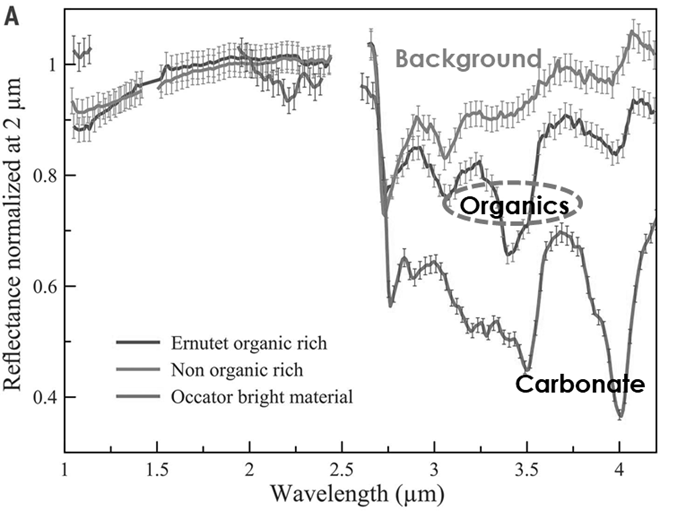
Fig 1 Ceres spectra of the organic-rich area in Ernutet crater (label “Organics”); of a background organic-poor area from a region southeast of Ernutet (label “Background”); and of Occator bright material (label “Carbonate”) [4].
In order to understand the organic chemical species and in particular their abundance on Ceres, laboratory studies were performed [7]. The importance of having a direct comparison between laboratory and remote sensing data can provide a further investigation clues to shed light on the origin and evolution of Ceres. Through this project, we intend to study, through dedicated experiments, the interaction between minerals, water, and organic concerning the environmental conditions of Ceres. Making a synergistic use of complementary and indispensable skills present within INAF (Italian National Institute of Astrophysics) laboratories we investigated a complex issue such as that concerning the origin and preservation of organic molecules on planetary surfaces. Within INAF, complementary and unique realities coexist which, thanks to joint and coordinated work, can give a new interpretation of the physical-chemical processes active on Ceres.
Project development and results
In this study, we prepare mixtures of materials resembling the Ceres surface composition [8, 9] adding organic molecules in order to:
(i) understand how organic molecules behave and eventually degrade on Ceres, in particular, how aliphatic molecules degrade by energetic processing with fast ions (keV-MeV) and UV photons [10, 11]. Moreover, the physico-chemical properties of the materials exposed to a flux of neutral atoms are investigated [12, 13].
(ii) evaluate the interaction between ammoniated minerals and simple organic molecules that may lead to the synthesis of complex compounds. In the presence of ultraviolet (UV) radiation, these minerals present on the surface of Ceres can show photocatalytic effects accelerating the photo-reactions, which generally destroy the original organic molecule and in the synthesis of new complex organic molecules [14].
(iii) evaluate the role of minerals in the protection or degradation of organic compounds. Some studies indicated a fundamental role of clays in the catalysis and preservation of organic materials [15]. Ceres is rich in clays and other hydrated minerals, making the interactions with the observed organics of particular interest.
The project is carried out by several INAF institutes and laboratories. In detail: INAF-IAPS Istituto di Astrofisica e Planetologia Spaziali prepared the analog mineral mixtures taking into account the compositional information gained by VIR observations. INAF - Osservatorio Astrofisico di Arcetri subsequently doped the mixture with several organic investigating UV photostability in Ceres analog conditions and the influence of temperature. INAF -Osservatorio Astronomico di Capodimonte studied irradiation with atoms and temperature effect while INAF - Osservatorio Astrofisico di Catania performed irradiation with fast ions. Finally, results of laboratory measurements were compared with data obtained by VIR instrument onboard Dawn mission.
Acknowledgements
This work is support by INAF Main Stream programme, grant 1.05.01.86.08 Evoluzione ed alterazione del materiale organico su Cerere (ref. Maria Cristina De Sanctis).
References
[1] De Sanctis et al., 2016, Nature 536, 54–57
[2] Marchi et al., Nature Astr., 2019
[3] De Sanctis et al., 2011, Space Science Reviews 163, 329-369.
[4] De Sanctis et al., science 2017 355, 719
[5] Pieters et al., 2018, Meteoritics and Planetary Science 53 (9), 1983-1998
[6] De Sanctis et al., 2019, 482 (2), 2407–2421
[7] Vinogradoff et al., 2021
[8] Ferrari et al., 2019, Icarus 321, 522-530
[9]De Angelis et al., 2021 JGR Planets doi: 10.1029/2020JE006696
[10] Baratta et al. 2002, A&A, 384, 343-349
[11] Brucato et al. 2006, A&A, 455, 395-399
[12] Mennella et al. 2003, ApJ, 587, 727-738
[13] Palumbo et al 2004, Ad. Sp. Res., 33, 49-56
[14] Fornaro et al. 2013, Icarus, 226(1), 1068–1085
[15] Fornaro et al 2018, Astrobiology, 18, 989-1007
How to cite: Poggiali, G., De Sanctis, M. C., Brucato, J. R., Ferrari, M., De Angelis, S., Palumbo, M. E., Baratta, G., Mennella, V., Fulvio, D., Popa, C., Strazzulla, G., and Sciré, C.: Evolution and alteration of organic material on Ceres, a pathway towards the understanding of complex geological and chemical history of a wet small body, Europlanet Science Congress 2021, online, 13–24 Sep 2021, EPSC2021-723, https://doi.org/10.5194/epsc2021-723, 2021.
Several lines of evidence indicate that most of the smaller asteroids (< 1 km) consist of granular material loosely bound together primarily by self-gravity; these are commonly called rubble piles [1]. While the strength of these rubble piles is valuable information on their origin and fate, it is still debated in the literature [2]. Therefore, we have started a laboratory measurement campaign on simulated asteroid regolith, studying the impact of several factors on material strength, such as grain size, size mixtures, and surface properties. In the work presented here, we focus on fine-coarse mixtures and the influence of the fraction of fines on the sample strength. Computer simulations suggest that the increase in the ratio of fine grains to coarse grains will increase the strength of the sample in all configurations [3]. In a series of table-top measurements, we have determined sample compression and shear strengths for various fine-coarse mixtures. We used confined setups (less than 10cm in length) to measure the strength of the material in constricted environments such as an asteroid’s core and unconfined setups (greater than 10cm in length) to simulate open environments such as the surface of an asteroid.
Using CI Orgeuil high fidelity asteroid soil simulant [4], we performed three measurement types to determine the strength of our samples. Samples of regolith were created by measuring percentage by volume amounts of both coarse and fine grains into the measurement container. We prepared coarse grains in two size distributions, mm-sized (Figure 1) and cm-sized. The fine fraction was composed of grains sieved between 100 and 250 µm. A shear box setup was used to obtain shear yield measurements which in turn provided values for the Angle of Internal Friction (AIF), bulk cohesion, and tensile strength of the samples. A compression setup was used to measure values for the Young’s Modulus (YM) in both confined and unconfined samples. The third setup measured the Angle Of Repose (AOR), the steepest angle of descent relative to the horizontal plane to which a material can pile before collapse. From the AOR, we determined the coefficient of friction of each sample.
For compression and AOR measurements, we find that the strength of the coarse grain samples increases with the addition of a fine fraction (Figure 2, left). These findings are intuitive and support the results from computer simulations. However, we find that the increase of the fine fraction in a sample of coarse grains does not consistently increase the sample shear strength. With increasing fine fractions, the AIF and bulk cohesion (Figure 2, right) of the mixed samples decrease (until a point of saturation). This could be indicative of the fine grains acting as a lubricant as the larger grains move across each other, aiding rolling and reducing interlocking strength.
Our findings suggest that in the case of the surface of an asteroid, the presence of fine grains does indeed increase the strength of coarse regolith material. However, fine grains in the regolith sublayers or the asteroid interior will reduce material strength due to grain interlocking and ease disruption. Therefore, rubble piles that are depleted in fine grains will have higher internal strength compared to those composed of grain size distributions that include sub-mm sized particles.
[1] Walsh, K.J., 2018. Rubble pile asteroids. Annual Review of Astronomy and Astrophysics, 56, pp.593-624.
[2] Holsapple, K., 2020. Main Belt Asteroid Histories: Simulations of erosion, cratering, catastrophic dispersions, spins, binaries and tumblers. arXiv preprint arXiv:2012.15300.
[3] Sánchez, P. and Scheeres, D.J., 2014. The strength of regolith and rubble pile asteroids. Meteoritics & Planetary Science, 49(5), pp.788-811.
[4] Metzger, P.T., Britt, D.T., Covey, S., Schultz, C., Cannon, K.M., Grossman, K.D., Mantovani, J.G. and Mueller, R.P., 2019. Measuring the fidelity of asteroid regolith and cobble simulants. Icarus, 321, pp.632-646.
How to cite: Cox, C., Brisset, J., Partida, A., Madison, A., and Bitcon, O.: Mechanical properties of fine-coarse grain mixtures of asteroid regolith, Europlanet Science Congress 2021, online, 13–24 Sep 2021, EPSC2021-454, https://doi.org/10.5194/epsc2021-454, 2021.
Ammonium minerals have been proposed to be present in variable percentage on icy planetary bodies such as Enceladus, Ceres, Pluto and its satellites and their presence is evident also in others celestial bodies. The presence of these compounds is related to the raise of ammonium (NH4+) salts from the interior of the icy planetary body, where oceans are likely located, due to cryovolcanism activity mixed with ice. De Sanctis et al. (2020) suggest that the presence of ammonium bicarbonate and/or ammonium chlorides on Ceres’s surface is a trace of the recent ascent of deep brine. In fact, some authors (e.g., Castillo-Rogez, 2020) proposed the presence of an early ocean on the subsurface of Ceres as deep brine. The Virgil Fossae on Pluto show evidence of recent cryovolcanism activity and ammonia spectral signature (Cruikshank et al., 2019) revealed by the analysis of data collected by New Horizons from the Linear Etalon Imaging Spectral Array (LEISA) (Schmitt et al., 2017). Recently, several studies (Cook et al., 2018; Dalle Ore et al., 2019) modelled the surface composition of Nix, Hydra and Kerberos, Pluto’s moons, using ammoniated salts as end members: NH4Cl, NH4NO3 and (NH4)2CO3. The identification of these minerals on the surface can give information about internal composition/dynamics and potential habitability of icy bodies. Among the tested samples, several hydrated and anhydrous ammonium compounds undergo phase transitions under specific temperature conditions. For these reasons, these minerals at cryogenic conditions can experience variations in resistivity, electrical conductivity as well as other mechanical properties, that can affect the internal dynamics, cryovolcanism and buoyancy of celestial bodies.
This study focuses on a series of selected minerals, as sulfates, phosphates, aluminates and borates, and is aimed at understanding how (1) different anionic groups, (2) the amount of water, (3) the occurrence of low temperature phase transitions and (4) different grain-size affect the absorption bands parameters of the ammonium bearing minerals. As the experimental data to interpret the remote sensing data, available nowadays for these systems, are usually restricted to small spectral ranges and collected only at room temperature, we performed reflectance spectroscopy analyses in the near-infrared (NIR) region (1-5 μm) in a temperature range from 298K to 60K with specific temperature steps for samples characterised by phase transitions. The reflectance spectra of the samples were measured under cryogenic conditions representative of real planetary surfaces. In addition, ammonium compounds were sieved in three different grain size ranges: 36-80, 80-125 and 125-150 μm. Each grain-size was measured at room temperature. X-ray diffraction analyses were performed on the samples before and after thermal treatments.
We measured natural and synthetic ammonium bearing minerals; sal-ammoniac NH4Cl, mascagnite (NH4)2SO4, ammonium bicarbonate (NH4)HCO3, ammonium nitrate NH4NO3, ammonium carbonate (NH4)2CO3, ammonium phosphate monobasic (NH4)H2PO4, larderellite (NH4)B5O7(OH)2·H2O, struvite(NH4)MgPO4·6H2O and tschermigite (NH4)Al(SO4)2·12H2O using using the SHINE spectro-gonio radiometer of the Cold Spectroscopy Facility (https://cold-spectro.sshade.eu) at IPAG equipped with a simulation chamber to control the temperature of the minerals. Some of them undergo structural transformations at cryogenic temperature: i.e., mascagnite shows phase transitions at 223 K which involve a changing from space group Pnam at room temperature to Pna21, with ferroelectric behaviour related to a stronger hydrogen bond, whereas tscermigite presents low temperature transitions at 76 K.
Reflectance spectra of anhydrous samples show well defined absorption features in the 1-2.5 µm range due to NH4+ groups overtones and combinations. The bands located at 1.3 (2ν3 + ν4) and 1.56 (2ν3) µm could be useful to discriminate these salts. The reflectance spectra of water-rich samples show H2O fundamental absorption features, overlapped to the NH4+bands, in the area from 1 to 2.8 μm and over 3 μm the spectra show a minor number of peaks. The parameters (area, depth and FWHM) of several absorption bands change in relation to the low temperature conditions and different grain size. In detail, the low temperature spectra compared to the room temperature ones reveal fine structure displaying more specific and defined absorption bands. The different granulometry affects mainly the bands area and depth. Moreover, we notice as the grain size becoming larger, the value of area and FWHM (full width half maximum) increase. Samples mascagnite (NH4)2SO4, sal-ammoniac NH4Cl, ammonium phosphate (NH4)H2PO4, tschermigite (NH4)Al(SO4)2·12(H2O) and ammonium nitrate NH4NO3 are characterized by phase transitions at low temperature and in some cases showed clear and very interesting spectral bands variations during cooling, indicating that a phase transition occurred. In these minerals, the detected phase transitions are characterized by a progressive deepening and shift toward shorter wavelength whit an abrupt change in depth of the sensitive bands.
In the analysed temperature range, like that can be found on the surface of large icy bodies, the ammonium minerals undergo different evolutions. In some cases, phase transformations generate important variations in the structural configuration that reflect on the characteristics of the band’s parameters and shape. In this scenario, the behaviour of the studied minerals at low temperature is very interesting for the remote sensing identification. These collected cryogenic data, with a carefully analysis of NH4+ absorption features, could be used to the detection of these salts on the surfaces of planetary bodies. The presence of ammonium minerals in the ice shell could influence the dynamics of icy satellites, especially if they are subject to phase transformations.
- Castillo-Rogez, J. (2020). Future exploration of Ceres as an ocean world. Nature Astronomy, 4(8), 732-734.
- Cook, J. C. et al., (2018). Composition of Pluto’s small satellites: Analysis of New Horizons spectral images. Icarus, 315, 30-45.
- Cruikshank, D. P. et al., (2019). Recent cryovolcanism in virgil fossae on Pluto. Icarus, 330, 155-168.
- Dalle Ore, C. M. et al., (2019). Detection of ammonia on Pluto’s surface in a region of geologically recent tectonism. Science advances, 5(5), eaav5731.
- De Sanctis, M. C et al., (2020). Fresh emplacement of hydrated sodium chloride on Ceres from ascending salty fluids. Nature Astronomy, 4(8), 786-793.
- Schmitt, B. et al., Physical state and distribution of materials at the surface of Pluto from New Horizons LEISA imaging spectrometer. Icarus, 287, 229-260.
How to cite: Fastelli, M., Comodi, P., Zucchini, A., Schmitt, B., Beck, P., and Poch, O.: VIS-NIR analysis at low temperature and different grain size of ammonium bearing minerals: a tool to improve the knowledge of icy planetary bodies surface., Europlanet Science Congress 2021, online, 13–24 Sep 2021, EPSC2021-313, https://doi.org/10.5194/epsc2021-313, 2021.
Introduction
The determination of surface properties of Solar System rocky bodies is a fundamental step in the interpretation of remote-sensing data from planetary missions. Estimating the surface temperature from remotely sensed spectroscopic data data is generally performed by applying models and inversions to infrared spectra. Such models have been used in the past to estimate the surface temperature of planetary bodies like Mars and Ceres (e.g. Pollack et al., 1990; Tosi et al., 2016). In some specific case, namely water ice, the absorption bands in the near infrared range have been calibrated in position (Fink and Larson, 1975; Grundy and Schmitt, 1998; Mastrapa et al., 2009) and their shift with respect to laboratory P-T conditions can be used to infer the surface temperature of icy satellites if these are relatively free from contaminants, or of ice outcrops on smaller bodies (see e.g. Raponi et al., 2018, for Ceres). Other non-water-ice materials can be in principle used as temperature proxy, useful for an independent estimation of the surface temperature of rocky bodies. Phyllosilicates for example are characterized by diagnostic OH absorption bands in the 1-2.8 μm-region, and these have been detected on Mars (Bibring et al., 2005; Ehlmann and Edwards, 2014) as well as on asteroids (De Sanctis et al., 2015; Hamilton et al., 2019; Kitazato et al., 2019) by telescopic and space missions. Nevertheless, the absorption of water hiddens or strongly affects the structural OH absorption in the IR, thus other non-hydrated materials are needed in order to be related to temperature changes. Carbonates, also detected on bodies such as Mars (Ehlmann et al., 2009) or Ceres (De Sanctis et al., 2016) are good candidates for such a study, because they are characterized by a number of absorption bands that may be little to no affected by water; in particular the bands at 3.4-4 μm are outside the broad 3-μm water band. In previous laboratory studies carbonates reflectance spectra in the IR have been acquired at room temperature (Harner et al., 2015). In other laboratory studies a correlation between band position and temperature change has been showed for 3.4 and 4-μm bands (De Angelis et al., 2018) regarding the natrite, although measurements were carried out at low spectral resolution.
Methods
In this work we studied the IR spectral reflectance of a number of different carbonates, in the 3.2-4.6-μm range, at high spectral sampling and resolution, in a wide temperature range from 270 K down to 60 K. This set of measurements is part of a larger project, which aims at investigating also other classes of anhydrous minerals at high spectral resolution, in order to identify other valuable temperature proxies that can be useful in the interpretation of remote-sensing data from current and future planetary missions. We acquired spectra on six different types of carbonates, namely: calcite (CaCO3), dolomite (CaMg(CO3)2), magnesite (MgCO3), siderite (FeCO3), natrite (Na2CO3), malachite (Cu2(CO3)(OH)2). These materials cover a wide range of carbonates with different cations (Ca2+, Mg2+, Fe2+, Na+, Cu2+) thus allowing studying also the band variability due to the different chemical environment. Spectra were acquired with the setups of the Cold Surfaces Spectroscopy (CSS; https://cold-spectro.sshade.eu) facility at the Institut de Planétologie et d’Astrophysique de Grenoble (IPAG); measurements were performed with the SHINE Spectro-Gonio-Radiometer facility (Brissaud et al., 2004) equipped with a simulation chamber to control the sample temperature. The spectral sampling was 3 nm, corresponding to a spectral resolution < 8 nm. This facility uses a monochromator as a light dispersion element. All spectra were acquired at standard conditions of illumination (i = 30°) and emission (e = 0°). Spectralon and Infragold (Labsphere ©) were used as reference targets.
The materials have been analyzed in the form of fine powders, each mineral having been ground and dry-sieved at grain size below 50 μm.
Preliminary results and Conclusions
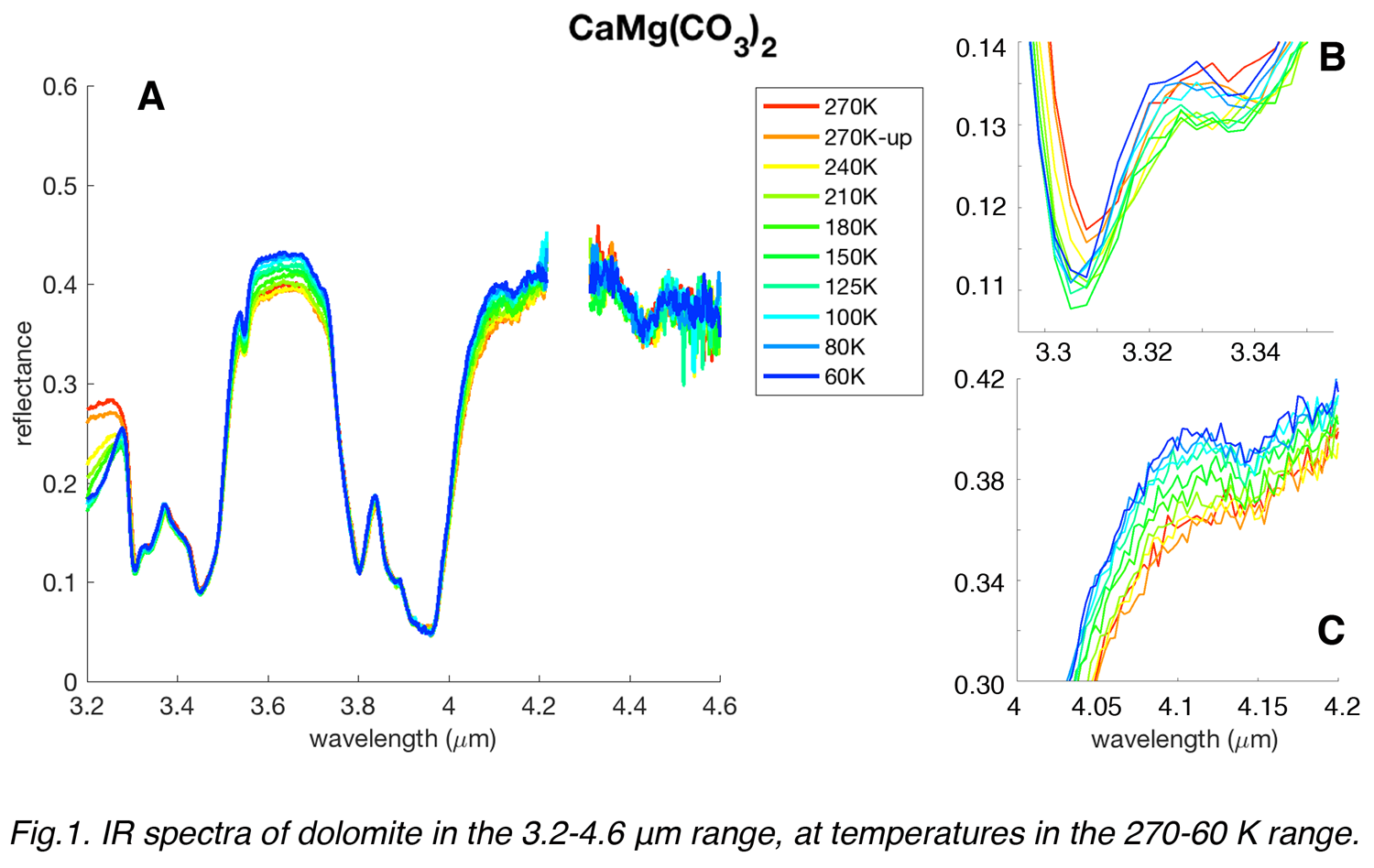
Spectra of a dolomite sample are shown in Fig.1. Several changes can be seen in the spectra as the temperature is lowered from 270 to 60 K. In Fig.1A the overall 3.2-4.6 μm-spectra are shown. The reflectance level at continuum becomes higher for wavelengths beyond 3.5 μm; below 3.3 μm a decrease of reflectance could be related to some minor amount of water contained in the sample, or to frost condensation in the chamber. In Fig.1B a closeup on the first minimum of the carbonate 3.4-μm band is shown. The position of the first minimum, located at about 3.31 μm at 270 K, seems to shift towards shorter wavelengths as the temperature decreases, by roughly 6-7 nm. In Fig. 1C a closeup on the 4-4.2-μm region is displayed. A very weak band, that is quite unrecognizable at 270 K, becomes clearer and definite as the temperature decreases to 60 K, located at 4.15 μm.
All these subtle changes in spectral bands positions as well as small bands appearing at low temperatures could be in principle detectable by high-resolution spectrometers.
Future work will deal with the detailed spectral analysis of band parameters of all the carbonate samples, as well as with the investigation of other anhydrous materials.
References
Bibring J.-P. et al., 2005. Science 307, 1576
De Angelis, S., et al., 2018. Icarus 317, 388-411
De Sanctis M.C., et al., 2015. Nature, 528, 241-244
De Sanctis M.C., et al., 2016. Nature,536, 54-57
Ehlmann B.L., et al., 2008. Science, 322, 1828-1832
Ehlmann B.L. and Edwards C.S., 2014. Annual Review of Earth and Planetary Science, 42, 291-315
Fink U. and Larson H.P., 1975. Icarus, 24, 411-420
Grundy W.M. and Schmitt B., 1998. Journal of Geophysical Research, Vol.103, N.E11, Pages 25,809-25,822
Hamilton V.E., et al., 2019. Nature Astronomy
Harner P.L. and Gilmore M.S., 2015. Icarus 250, 204-214
Kitazato K., et al., 2019. Science, 364, 272-275
Mastrapa R.M. ,et al., 2009. Astrophysical Journal 701, 1347–1356
Pollack J.B., et al., 1990. Journal Of Geophysical Research, Vol. 95, No.B9, Pages 14,595-14,627
Raponi A. et al., 2018. Science Advances 4
Tosi F., et al., 2016. 47th Lunar and Planetary Science Conference, abstract #1883
How to cite: De Angelis, S., Schmitt, B., Beck, P., Carli, C., Poch, O., Brissaud, O., and Tosi, F.: High spectral resolution / low-temperature IR study of carbonates, Europlanet Science Congress 2021, online, 13–24 Sep 2021, EPSC2021-766, https://doi.org/10.5194/epsc2021-766, 2021.
Abstract
Silicates are the main constituent of volcanic terrains on terrestrial planets and other rocky bodies in the solar system [1]. Typically, these volcanic terrains are constituted by fragmented pyroclasts whose texture is often afanitic or porphyric rather than holocrystalline: this means that the fraction of crystalline material is less relevant than the fraction of amorphous, or glassy, material. Thus, it is of paramount importance to take into account amorphous silicate phases to explore the influence of glass/crystal ratio on the spectral response of volcanic rocks, to better interpret available and future remotely sensed spectra from past and future missions [2, 3]. Here we report the results of a study concerning mafic volcanic products which were synthesized in order to present different degrees of crystallinity: three basaltic melts were cooled at different rates to obtain different textures, from totally amorphous to crystalline. Finally, they were analysed by means of emissivity in the thermal-IR range at different temperatures.
Samples preparation
Samples were produced by melting two natural mafic rocks: a basalt (low-alkali mafic rock from Snake River Plain, USA) and a shoshonite (high-alkali mafic from Vulcano island, Italy) following a two-steps routine of crushing and melting [4, 5]. A third silicate melt was produced by mixing and melting oxides to resemble the composition of Nakhlite meteorite [6]. The three melts were cooled in three different ways (Fig. 1) to obtain nine different samples. In order to obtain pure, crystal-free glasses, melts were directly quenched from super-liquidus temperature (Black line Fig.1), whereas other, identical, melts were cooled down slowly (52-56 °C per hour) and then quenched at subliquidus temperatures of ca. 1100°C (red line Fig. 1) and 1000°C (yellow line Fig.1). Following this approach, we obtained 9 rocky samples from three melts, so that each sample was differing in both chemical composition and crystallinity.
|
Figure 1: The three cooling ramps used for the syntheses of the material. |
Samples analysis
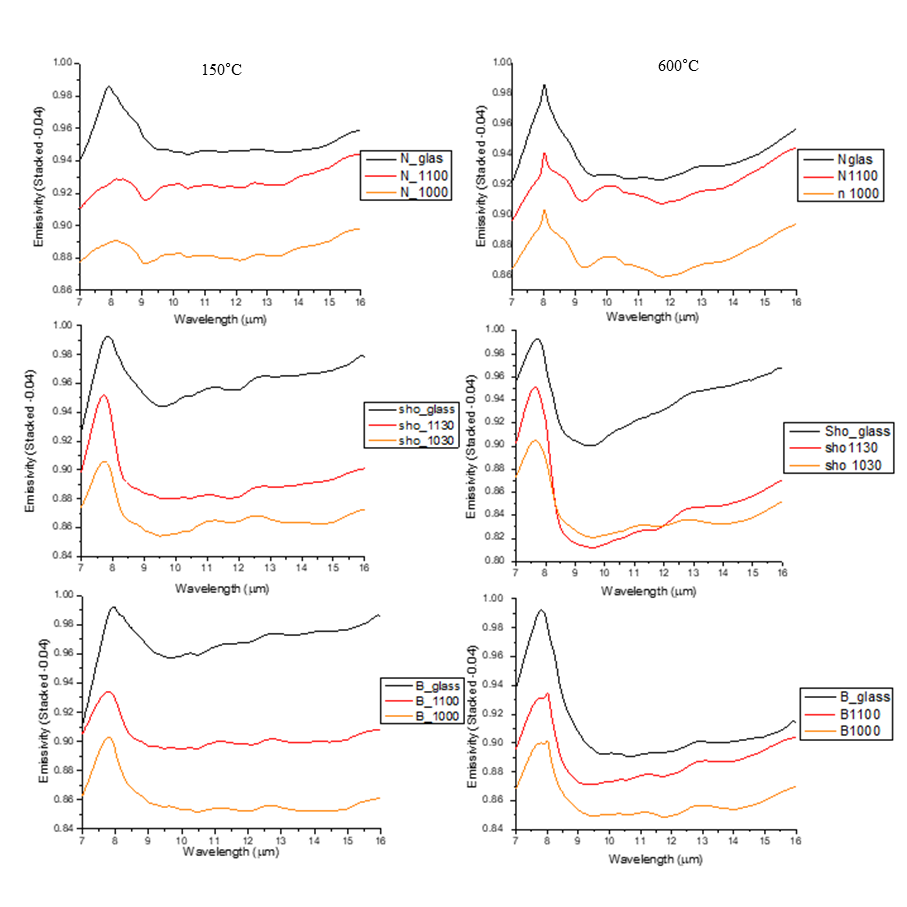
Samples were analysed using SEM, and spectroscopically characterized under different conditions: thermal-IR data have been acquired at the Planetary Spectroscopy Laboratory of the German Aerospace Center in Berlin (DLR), collecting the emitted thermal radiation for samples at different temperatures (150°C, 300°C, 450°C, 600°C; spectral range 5-16 μm) [7]. SEM imaging showed successful different degree of crystallinity for the three steps, which results in a different spectral response, visible in Figure 1.
By observing shape of spectra, crystalline Shoshonite and Basalt show similar shape to their relative amorphous but for a shoulder at 8.2-8.3 μm, whereas Nakhlite shows substantially different shapes for the three crystallinity steps (Fig. 2).
For what concerns the shift of the spectra, crystal-bearing products seem to show similar features at slightly lower wavelengths for Shoshonite and Basalt, whereas this trend is inverted for Nakhlite, probably due to different phases nucleating in different melts. Higher emissivity temperatures seem to homogenize the spectral response of samples with same chemical composition and different textural properties. These results provides further information on the spectral response of synthetised rock samples [2], that can be used for modeling of spectral information coming from rocky bodies in the Solar system
|
Figure 2: Spectra resulting from emissivity measurements performed on nine different products produced from three initial compositions (Nakhlite:N, Shoshonite:sho and Basalt:B). Emissivity was measured with samples at four different temperatures, two of which are here shown (150 and 600°C). |
References
[1] Namur, O. and Charlier, B. (2017). Silicate mineralogy at the surface of mercury.Nature Geoscience, 10(1):9.
[2] Pisello, A., Vetere, F. P., Bisolfati, M., Maturilli, A., Morgavi, D., Pauselli, C., ... & Perugini, D. (2019). Retrieving magma composition from TIR spectra: implications for terrestrial planets investigations. Scientific reports, 9(1), 1-13.
[3] Di Genova, D., Hess, K.-U., Chevrel, M. O., and Dingwell, D. B. (2016). Models for the estimation of fe3+/fetotratio in terrestrial and extraterrestrial alkali-and iron-rich silicate glasses using raman spectroscopy.
[4] Vetere, F., Iezzi, G., Behrens, H., Holtz, F., Ventura, G., Misiti, V., ... & Dietrich, M. (2015). Glass forming ability and crystallisation behaviour of sub-alkaline silicate melts. Earth-science reviews, 150, 25-44.
[5] Rossi, S., Petrelli, M., Morgavi, D., Vetere, F. P., Almeev, R. R., Astbury, R. L., & Perugini, D. (2019). Role of magma mixing in the pre-eruptive dynamics of the Aeolian Islands volcanoes (Southern Tyrrhenian Sea, Italy). Lithos, 324, 165-179.
[6] Treiman, A.H. (2005) The nakhlite meteorites: Augite-rich igneous rocks from Mars. Chemie der Erde 65, 203-270
[7] Maturilli, A., Helbert, J., Ferrari, S., Davidsson, B., and D’Amore, M. (2016). Characterization of asteroidanalogues by means of emission and reflectance spectroscopy in the 1-to 100-μm spectral range.Earth,Planets and Space, 68(1):113
How to cite: Pisello, A., Maturilli, A., Porreca, M., and Perugini, D.: Thermal-IR emissivity investigation on lab-made silicate rocks: implications for asteroidal and planetary studies., Europlanet Science Congress 2021, online, 13–24 Sep 2021, EPSC2021-722, https://doi.org/10.5194/epsc2021-722, 2021.
Introduction
In the research on the origin of life on our Planet, the possibility of a contribution played by organic substances of extraterrestrial origin, which may have reached our planet through micro-fragments of planetary and cometary origin, is being taken into consideration more and more seriously (Flynn et al., 2003). These particles may also provide the necessary thermal protection for thermolabile, life-related molecules, to the high temperatures reached during the first stages of the atmospheric entry processes. Particularly interesting is the size of the order of a tenth of a millimeter, which corresponds to a peak in the distribution of the material entering the atmosphere.
Physical models of the entry process of such grains have been available in the literature for many years. When the composition of most promising mineral phase is considered, the most interesting candidates are carbonates (mostly of Mg, Ca, and Fe) which have been associated, in meteorites and in cometary grains, with organic molecules (McKay et. al., 1996; Flynn et al., 2000; Pizzarello et al., 2006; Matrajt et al., 2012) for several reasons. Carbonates are very common in the Solar System. Outside the Earth, they have been identified on the surface of Mars (Ehlmann et al., 2008; Palomba et al., 2009; Wray et al., 2016), on Ceres (Rivkin et al., 2006; De Sanctis et al., 2016) and tentatively on other asteroids (Rivkin, 2009) as well as in cometary comas (Fomenkova et al., 1992; Lisse et al., 2006; Wirick et al., 2007). Since these astrophysical sites are thought to be the main sources of meteorites and micrometeorites, it is not surprising to find carbonates also in these small objects which reach our planet. In particular, Mg-, Ca-, and Fe/Mn-rich carbonate globules of putative biotic origin have been found in the Martian meteorite ALH84001 (McKay et al., 1996).
Carbonate decomposition
All the available studies of the atmospheric entry grains assume compositions corresponding to the bulk of meteorite bodies, i.e. silicates and metals. Only recently, carbonates have been taken in account as carriers in an astrobiological perspective (Bisceglia et al., 2017; Micca Longo & Longo, 2017; 2018). Carbonates are known to undergo decomposition in vacuum at temperatures of a few hundred °C (producing metal oxides and gaseous carbon dioxide) (L’vov, 1997; 2002). Therefore, during the atmospheric entry, the grains with carbonate composition are expected to be enriched in oxides and depleted of the initial carbonate amount. This process, being endothermic, can contribute to the thermal protection of associated organic matter. However, the kinetics of the decomposition under such conditions is not well understood. The decomposition model developed in Micca Longo & Longo (2017 and 2018) is based on a well-mixed and ideal solid mixture, and it allows a first evaluation of grains behavior during their passage through the Earth’s atmosphere. The Langmuir law allows to calculate the evaporation rate, per unit time and area, as stated in Bisceglia et al. (2017), in terms of the vapor pressure for the solid mixture carbonate/oxide.
Laboratory measurements
The kinetics of CO2 diffusion within this kind of grains is still a subject if study. A first attempt was done in Micca Longo et al. (2019) where an important role for the evolution of the model was given to the interpretation of laboratory experiments emulating the conditions during the atmospheric entry processes.
In this work, we report the laboratory measurements on Ca and Mg carbonates done to follow the diffusion process of CO2 within such materials. Powders were thermally processed under vacuum (10-4 and 10-5 mbar), for about 3.5 hours, at several temperatures by using a Carbolite furnace able to reach temperatures up to 1200°C. Infrared spectroscopy, gravimetry, Scanning Electron Microscopy, and Energy Dispersive X-ray analysis were involved to investigate spectral, morphological, and compositional modifications induced by thermal processing as done in Orofino et al. (2007), Blanco et al. (2011) and Micca Longo et al. (2019).
References
Bisceglia E., Micca Longo G., Longo S. (2017) International Journal of Astrobiology 16, 130.
Blanco A., et al. (2011) Icarus 213, 473.
De Sanctis M.C., et al. (2016) Nature 536, 54.
Ehlmann B.L., et al. (2008) Science 322, 1828.
Flynn G.J., et al. (2000) Bioastronomy 99, 213.
Flynn G.J., et al. (2003) Geochimica et Cosmochimica Acta 67, 4791.
Fomenkova M.N., et al. (1992) Science 258, 266.
Lisse C.M., et al. (2006) Science 313, 635.
L’vov B.V. (1997) Thermochimica Acta 303, 161.
L’vov B.V. (2002) Thermochimica Acta 386, 1.
Matrajt G., et al. (2012) Meteoritics & Planetary Science 47, 525.
McKay D.S., et al. (1996) Science 273, 924–930.
Micca Longo G., Longo S. (2017) International Journal of Astrobiology 16, 368.
Micca Longo G., Longo S. (2018) Icarus. doi: 10.1016/j.icarus.2017.12.001
Micca Longo G., et al. (2019) Geoscience. doi: 10.3390/geosciences9020101
Orofino V., et al. (2007) Icarus 187, 457.
Palomba E., et al. (2009) Icarus 203, 58.
Pizzarello S., Cooper G.W., Flynn G.J. (2006) Meteorites & the Early Solar System 2, 625.
Rivkin A.S. (2009) Division for Planetary Sciences Meeting Abstracts 41, Abstract #32.07.
Wirick S., et al. (2007) Lunar and Planetary Science Conference Proceedings 38, Abstract #1534.
Wray J.J., et al. (2016) Journal of Geophysical Research: Planets 121, 652.
How to cite: Mancarella, F., D'Elia, M., Orofino, V., Micca Longo, G., and Longo, S.: Experimental results of decomposition of Ca- and Mg- carbonates under conditions of interest to planetology, Europlanet Science Congress 2021, online, 13–24 Sep 2021, EPSC2021-456, https://doi.org/10.5194/epsc2021-456, 2021.
I. Introduction
Ices throughout the ISM are exposed to different energetic processes that trigger several reactions and change the com- position of the ice [1–3]. Particularly, ices in stars–forming regions can be subjected to the ultraviolet radiation that comes from the new born stars and triggers reactions in the ice. These ices are normally composed of H2O, CO, CO2, NH3 , methanol (CH3OH), and traces of other molecules. Irradiation–induced reactions in these ices are a source of complex organic molecules (COM) that might later feed the building blocks of planets or other bodies that are being formed. Methanol is one of the main constituents of interstel- lar ices, where its abundance can go up to 30% (with respect to water) [1, 3, 4]. With this in mind, we irradiated pure methanol ice deposited at 20 and 80 K, with UV radiation during different periods of time to evaluate the effect of fluence and temperature in the abundance of volatile COMs that formed.
II. Methods
All experiments were carried out in the VAHIIA set-up [5]. Briefly, the system consist of an ultrahigh vacuum chamber connected to a GC–MS through a pre–condensation loop. The latter consist of a stainless steel loop that is immersed in liquid nitrogen for the recovery of the volatile COMs coming from the vacuum chamber. This is connected to a custom–made group of valves that allows to recover volatile COMs for their injection in the GS–MS for identification. Species recovered were identified by comparing the chromatographic peak and mass spectra with the standard database, whose retention time and mass spectrum were obtained in the system under the same conditions as the experiments.
Pure methanol was deposited in a copper plated surface attached to the tip of the cryostat in the chamber at 20 K. Six periods of time were used: 15 and 30 min, 1, 3, 8 and 24 h, for evaluating different UV fluence. Each experiment consists of five layers of 0.2 mbar of pure methanol ice each, one on top of the other. Each of these layers is irradiated during the period of time under evaluation, with a UV flux of ∼1e13 photons s−1 cm−2, using a flowing H2 microwave–discharge lamp. Layers have been verified to be opaque to the UV photons, ensuring the layer(s) underneath the one being irradiated are not affected further. Once five layers are irradiated, under the same conditions, the chamber is warmed up to ∼ 300 K and volatiles are recovered with the injection of Argon for transferring the sample to the pre–condensation loop. Each experiment consist of 5 layers having received the same irradiation dose to obtain a larger quantity of products, and facilitate their detection and identification with the GC-MS. In addition, experiments were carried out at 80 K to evaluate the effect of the temperature on the abundance of volatiles formed. In this case we evaluated 30 min, 1 h and 24 h of irradiation.
III. Effect of fluence
23 molecules were identified after UV–irradiation of methanol ice. Figure 1 shows the absolute area under the total ion content curve of the molecules identified, as a function of the irradiation time. This quantity is a function of the abundance of the compound and is hereafter referred to as integrated TIC. Within functional groups, the same pattern is seen for molecules with different numbers of carbons. Aldehydes are the main functional group that forms. With the exception of formaldehyde, all of them have a similar increase up to 8h of irradiation and then even though the integrated TIC increases, the slope is smaller. Alcohols have a low yield and have a steady integrated TIC. Ethers are produced rapidly and reach high integrated TIC during the first 3-8 h but then, their formation is lower than its usage for the formation of more complex molecules and its integrated TIC drops. Dimethyl Ether (DME) has the highest integrated TIC throughout all experiments. Ketones are the main products with 4 and 5 carbons, and maintain a constant increase with the time of irradiation. Esters and ketones are the only two functional groups identified with up to 5 carbons in the chain. With the exception of methyl formate, esters have a similar pattern. During the first 3 h of irradiation the integrated TIC increases rapidly but between 3-8 h there is a recession. After, the increase is the highest.
At 8 h of irradiation several molecules display an inflection point. Aldehydes’s rate of production decreases (the integrated TIC is lower than expected), while the integrated TIC of esters and ketones is higher. Special cases are formaldehyde, DME and dimetoxymethane, who fall under the detection threshold after 24 h of irradiation tends to zero, which indicates they are the main reactants to form the more complex molecules.
Fig. 1. Integrated TIC of volatiles formed after UV–irradiation at 20 K. Aldehydes: blue, ethers: yellow, alcohol: black, ketones: green, esters: red.
IV. Effect of temperature
At 80 K there is a reduction in diversity and integrated TIC of products, and there is no common pattern to describe the functional groups as in section III. Propanol, DME and 1,3,5–trioxane are not identified at anytime. Aldehydes are identified only after 24 h of irradiation, except for isobutyraldeyde that appears at all times. Alcohols and Ethers have a lower yield. Ketones are still produced at all times with constant yield, which suggest an efficient mechanism of formation.
References
[1] S. Maity, R. I. Kaiser, and B. M. Jones, Physical Chemistry Chemical Physics, vol. 17,no. 5, pp. 3081–3114, 2015.
[2] P. de Marcellus, C. Meinert, I. Myrgorodska, L. Nahon, T. Buhse, L. L. S.d’Hendecourt, and U. J. Meierhenrich, PNAS, vol. 112, no. 4, pp. 965–970, 2015.
[3] D. Paardekooper, J.-B. Bossa, and H. Linnartz, Astronomy & Astrophysics, vol. 592, p. A67, 2016.
[4] A. Bergantini, S. Góbi, M. J. Abplanalp, and R. I. Kaiser, The Astrophysical Journal, vol. 852, no. 2,p. 70, 2018.
[5] N. Abou Mrad, F. Duvernay, P. Theulé, T. Chiavassa, and G. Danger, Analytical chemistry, vol. 86, no. 16, pp. 8391–8399, 2014
How to cite: Tenelanda-Osorio, L. I., Bouquet, A., Mousis, O., and Danger, G.: Effect of the UV flux and temperature on the formation of complex organic molecules in astrophysical ices, Europlanet Science Congress 2021, online, 13–24 Sep 2021, EPSC2021-239, https://doi.org/10.5194/epsc2021-239, 2021.
The implantation of reactive charged species within low-temperature solids is relevant to astrochemistry and may lead to physico-chemical changes within the solid, such as the formation of new molecules which incorporate the projectile. We have performed the high-fluence (>1016 ions cm–2) implantation of S+ into CO, CO2 and H2O ices at 20 and 70 K. Our results show that implantation into CO and CO2 results in the formation of SO2 at 20 K, although no evidence of SO2 was observed at 70 K. Implantation into H2O yields H2SO4 hydrates. These results are applicable to Europa; one of the Galilean moons of Jupiter.
How to cite: Mifsud, D. V., Kaňuchová, Z., Herczku, P., Juhász, Z., Kovács, S. T. S., Sulik, B., Hailey, P. A., Traspas Muiña, A., Ioppolo, S., McCullough, R. W., and Mason, N. J.: S+ Implantation in Oxide Ices: Relevance to Europa, Europlanet Science Congress 2021, online, 13–24 Sep 2021, EPSC2021-247, https://doi.org/10.5194/epsc2021-247, 2021.
During its two years of operation on the comet 67P/Churyumov-Gerasimenko (67P), the Rosetta/OSIRIS instrument had observed numerous bright spots on the comet surface, with flat spectra in the visible (VIS) range that indicates the presence of water ice or other volatiles [1][2][3]. Towards the end of the mission in June – September 2016, a number of bright spots with an unsual negative VIS slope were detected in shadowed areas. Similar to the "blue" spots, frost was only observed on comet 67P in the Hapi region prior to the 2015 perihelion [4] but more widespread on the comet surface after that [5]. This correlation prompts us to investigate frost at different thicknesses, in which we produce and measure frost with the use of the SHINE equipment in IPAG.
Frost is produced by exposing a cold sample holder covered in black tape to the open air, which allows atmospheric vapor to condense on top. The sample holder can be cooled simultaneously with frost disposition via liquid nitrogen; or it can be placed inside a sealed cold chamber e.g. a freezer, and the frost condensation occurs after removing the sample holder from the cold chamber and results in the formation of porous "needles" structures. After frost production, the sample holder is transferred inside the pre-cooled SHINE chamber in order to obtain spectra. The inner chamber is kept at approximately 200 K and atmospheric level pressure throughout each acquisition in order to stabilize the frost, and frost thickness can be reduced by operating the vacuum pump for a few minutes. All measurements are conducted with the same observational geometry: incidence i = 0, emergence e = 30° and azimuth a = 0.
Our spectra feature show absorption bands at approximately 1.5 µm, 2 µm and 3 µm and also 1.65 µm in thicker frost samples (see Fig. 1). The water bands deepen with increasing frost thickness, in which the ~3 µm band is the first to appear with very thin frost layer (Fig. 1b) due to its highest sensitivity to water ice presence [6] and reaches saturation as soon as the frost layer is thick enough for the other two bands at 1.5 µm and 2 µm to appear. The Fresnel peak at ~3.1 µm is present in all spectra (except for the "pure" black tape"), indicating that the produced frost is composed of crystalline ice whose grain size is at least a few microns [7]. The ~1.65 µm peak is also a crystalline ice indicator, and its weak presence in our spectra can be explained by its high susceptiblity to temperature [8].
Fig. 1 also shows that frost becomes more spectrally blue in the VIS range with increasing thickness, where the thickest frost specimen (Fig. 1a) has a linear-fitted slope of -3.36%/ (100 nm) in the 540 – 880 nm range, similar to a few "blue" spots on the comet whose spectral slope value is approximately -3%/ (100 nm) in the 535 – 882 nm range. A possible explanation for the blue VIS slope is the presence of very large water ice grain i.e. mm-sized or larger, as shown in simulated water ice spectra by Hapke modelling [9]. These results appear to support our hypothesis that frost could be a possible cause of the spectrally blue bright spots, and future experiments will seek to investigate the aforementioned matter further.
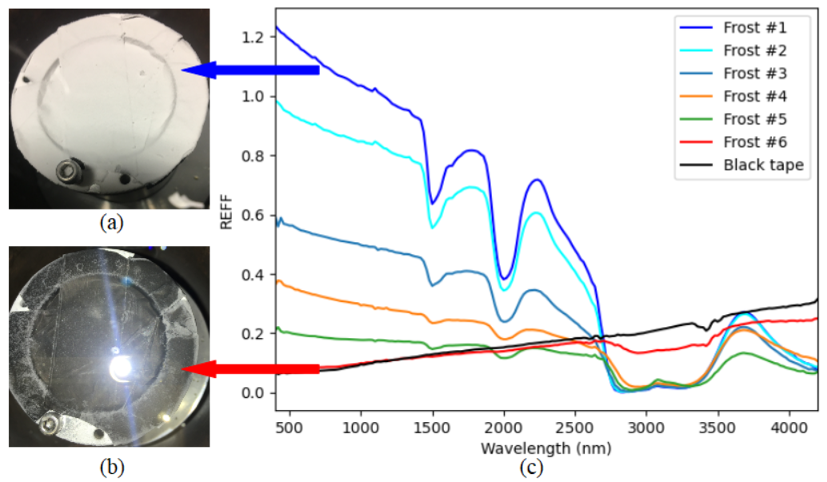
Fig. 1: The image of a thick frost sample (a) and a thin frost sample (b) inside the SHINE chamber prior to measurement, alongside the spectra of frost at different thicknesses (c). The (a) and (b) samples correspond to the dark blue line and red line, respectively
References: [1] Barucci et al., 2016, A&A 595, A102; [2] Oklay et al., 2017, MNRAS 469, S582–S597; [3] Deshapriya et al., 2018 A&A 613, A36; [4] De Sanctis et al., 2015, Nature 525, 500–503; [5] Fornasier et al., 2016, Science 354, 1566; [6] Clark & Lucey., 1984, JGR 89, 6341-6348; [7] Hansen & McCord., 2004, JGR 109, E01012; [8] Grundy et al., 1999, Icarus 142, 536–549 (1999); [9] Raponi et al., 2016, MNRAS 462, S476–S490
How to cite: Hoang, H. V., Quirico, E., Fornasier, S., Poch, O., Beck, P., and Schmitt, B.: Frost on dark surfaces: Finding a possible link between spectrally blue spots and frost on the surface of comet 67P/Churyumov-Gerasimenko, Europlanet Science Congress 2021, online, 13–24 Sep 2021, EPSC2021-261, https://doi.org/10.5194/epsc2021-261, 2021.
Ices are widely present in the cold regions across the Universe, for instance, in the interstellar medium as mantles on interstellar and circumstellar dust and on the surfaces of small bodies of the Solar System - beyond the distance around 3-5 AU known as the “snowline" (i.e. at temperatures below 150-170 K). The continuous energetic processing of icy objects in the Solar System induces physical and chemical changes within the ice. Laboratory experiments that simulate energetic processing (ions, photons, and electrons) of ices are therefore essential for interpreting and directing future astronomical observations.
Here we provide vacuum ultraviolet (VUV) and UV-Vis photoabsorption spectroscopic data of pristine and energetically processed (electron irradiated) space-related ices. Experiments were performed using a custom-made Portable Astrochemistry Chamber (PAC), which has a base pressure of 10-9 mbar. Photoabsorption spectra of ices were measured at the AU-UV beam line of the ASTRID2 synchrotron light source at Aarhus University in Denmark (see Eden et al. 2006; Palmer et al. 2015). We present the results of three series of experiments: one dedicated to the study of nitrogen- and oxygen-rich ices (Ioppolo et al., 2020); the other one to the spectroscopic study of carbonic acid as formed and destroyed under conditions relevant to space (Ioppolo et al., 2021); and the third one to the study of photoabsorption spectra of O2 ice, both pure and mixed with other species (Migliorini et al, 2021).
Results are discussed in light of their relevance to various astrophysical environments, e.g., the icy moons of Saturn and Jupiter. Laboratory VUV-UV-vis spectra of ices can help their future identification on the surface of icy objects in the Solar System by the upcoming Jupiter ICy moons Explorer mission and on interstellar dust by the James Webb Space Telescope spacecraft.
This research was partly supported by the Italian Space Agency (Grant ASI-INAF n. 2018-25-HH-0).
REFERENCES:
Eden, S., Limão-Vieira, P., Hoffmann, S. V., & Mason, N. J. 2006, Chem. Phys., 323, 313
Ioppolo, S., Kanuchova Z., James, R.L., Dawes, A., Jones, N.C., Hoffmann, S.V., Mason, N.J., Strazzulla, G. 2020, Astron. Astrophys. 641, A154
Ioppolo, S., Kanuchova Z., James, R.L., Dawes, A., Ryabov, A., Dezalay, J., Jones, N.C., Hoffmann, S.V., Mason, N.J., Strazzulla, G. 2021, Astron. Astrophys. 645, A172
Migliorini, A., Kanuchova Z., Ioppolo, S., Jones, N.C., Hoffmann, S.V., Tosi, F., Piccioni, G., Barbieri, M. 2021, Icarus, submitted
Palmer, M. H., Ridley, T., Hoffmann, S. V., et al. 2015, J. Chem. Phys., 142, 134302
How to cite: Ioppolo, S., Kanuchova, Z., James, R. L., Dawes, A., Ryabov, A., Dezalay, J., Jones, N. C., Hoffmann, S. V., Mason, N. J., Strazzulla, G., Migliorini, A., Tosi, F., Piccioni, G., and Barbieri, M.: Vacuum ultraviolet photoabsorption spectroscopy of space-related ices, Europlanet Science Congress 2021, online, 13–24 Sep 2021, EPSC2021-580, https://doi.org/10.5194/epsc2021-580, 2021.
Introduction
The studied molecule belongs to cyclic hydrocarbons with one nitrogen atom. The molecule is found, for example, in nicotine and is also found in B vitamins, which are important for cell metabolism [1]. Pyridine is also used as a solvent or precursor for agrochemicals in fertilizers [2]. Pyridine attracts attention not only due to its industrial and biological consequences, but also due to astrochemistry. B vitamins have also been found on the surface of meteorites [3]. Pyridine was for example identified in the methanol extract of the Murchison meteorite, which is probably formed by the reaction of NH3 and aldehydes. The identification was done by liquid chromatography and mass spectrometry. This is one of the reasons why a thorough knowledge of the basic physical and chemical properties of this molecule is important.
Experiment
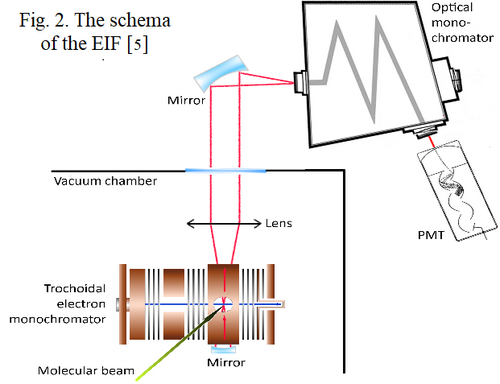
For investigation of electron induced processes of molecules an experiment with crossed electron and molecular beam was used. The sample (C5H5N, CAS: 110-86-1, purity: 99.8 %, Sigma Aldrich) is in liquid state in laboratory conditions and its vapors were introduced into the reaction chamber through molecular beam source (MBS) using capillary to generate effusive beam. The electrons were produced by trochoidal electron monochromator (TEM) generating an electron beam with a well specified energy with an energy resolution 300 meV FWHM. The products of these reactions were analyzed by Crossed Electron Molecular Beams Ionization Apparatus (CEMBIA) [4]. and Electron Induced Fluorescence Apparatus (EIF) [5].
In the case of the CEMBIA experiment, the ions generated by the electron-molecular collision are directed by an electric field to a quadrupole mass spectrometer (QMS), where they are separated based on their mass to charge ratio. Ions that pass through the QMS are detected by the channeltron (CEM). In the case of the EIF experiment, the photons emitted during the deexcitation of the excited reaction products pass through the optical system from the vacuum chamber to the optical monochromator and are detected by a photomultiplier operating in the photon counting mode.
Results
Using CEMBIA apparatus we have observed electron ionization and dissociative electron ionization of pyridine by electron impact. In the Fig. 3. the mass spectrum of the pyridine molecule measured at an electron energy of 70 eV is shown. The parent positive ion, denoted P+, weighs 79 amu. At 80 amu there is the isotope of the molecule. Subsequently, we see the dissociation of pyridine into fragments. Firstly, the hydrogen is sequentially dissociated from the parent. Subsequently, a very nice sequential carbon dissociation is seen, starting from a mass at 74 amu with the C5N+ product. Subsequently, additional carbon is likely to dissociate to the C4N+ product at 62 amu. Sequential dissociation probably continues through masses and products at 50 amu C3N+, 38 amu C2N+, 26 amu CN+ up to a mass at 14 amu, where we detected a fragment of the N+ nitrogen atom. Similar dissociations are intertwined across the spectrum. We were mainly interested in two fragments, C+ (mass 12) and CH+ (mass 13). The reason is the fluorescence spectrum measured by EIF apparatus in which we see the transitions associated with the positive ions C+ and CH+. The fluorescence emission spectrum of the pyridine molecule induced by monochromatic electrons is shown in Fig 4. In the spectrum we can see CH+ at the wavelength of 351 nm and 423 nm and C+ at the wavelength of 391.75 nm. Other detected products are not ionized. The fluorescence spectrum is measured at an interacting electron energy of 70 eV. The measurement range of the fluorescence spectrum is from 200 to 700 nm, with no signal being detected from 200 to 310 nm.
In the range from 525 to 700 nm, we detected only hydrogen Balmer series Hα line. The hydrogen Balmer series is also observed and identified. In Fig 5. we show an illustrative example of a 2D spectral map of the fluorescence cross section. The 2D map is made by measuring spectra in the range from 200 to 700 nm in steps of 0.1 nm for a constant energy of interacting electrons per measurement. The measurements are repeated in 5 eV steps ranging from 15 eV up to 100 eV.
Acknowledgments
This work was supported by projects APVV-19-0386 and VEGA 1/0489/21. This project has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No 692335.
References
[1] I. Linert, M. Zubek, Eur. Phys. J. D (2016) 70: 74
[2] M. A. Smialek and col., Eur. Phys. J. D (2016) 70: 42
[3] Y. Yamashita, H. Naraoka, Geochemical J. (2014) 48, 519-525
[4] M. Lacko, P. Papp and Š. Matejčík, J. Chem. Phys. (2018) 148, 214305.
[5] M. Danko and col., Plasma Sources Sci Technol (2016) 25, 065007
How to cite: Blaško, J., Országh, J., Stachova, B., Papp, P., and Matejčík, Š.: Excitation and ionization processes of Pyridine molecule induced by low-energy electron impact, Europlanet Science Congress 2021, online, 13–24 Sep 2021, EPSC2021-28, https://doi.org/10.5194/epsc2021-28, 2021.
The Universe is molecular in nature, as over 200 molecules have been detected in the gas-phase in the interstellar and circumstellar medium (ISM/CSM). Starting from molecular hydrogen, many species including H2O, CO2, NH3, CH4, CH3OH, and other complex organic molecules (COMs) have been shown to be formed efficiently on the surface of interstellar ice grains throughout the star-formation process. Interstellar ices are believed to be the main carriers of prebiotic molecules that have been included in the outer Solar System’s ice objects such as moons, comets, and Kuiper Belt Objects (KBOs). Therefore, understanding how COMs form and evolve in space is of pivotal importance to study the potential link between species in space and life on Earth.
COMs like the isomers of C2H4O2, i.e., glycolaldehyde (HCOCH2OH), acetic acid (CH3COOH), and methyl formate (HCOOCH3), have been observed abundantly around the Galactic centre, in dark clouds, and hot cores of the interstellar medium (ISM), as well as in some comets of the Solar System (e.g., Favre et al. 2011; Bockelee-Morvan et al. 2000). However, their exact gas-grain formation and destruction pathway is still unclear (Balucani et al. 2015). According to El-Abd et al. (2019), the observed column densities of methyl formate and acetic acid are well-correlated, and are likely simply tracking the relative total gas mass in star forming regions. Methyl formate and glycolaldehyde, however, display a stark dichotomy in their relative column densities. The latter finding implies that different formation/destruction routes are at play for the three isomers.
To date, there is a strong laboratory evidence for an efficient production of glycolaldehyde, methyl formate, and acetic acid in the ISM through energetic processing of methanol-rich interstellar ices (Gerakines et al. 1996; Bennett and Kaiser 2007; Oberg et al. 2009; Modica and Palumbo 2010; de Barros et al. 2011; Modica et al. 2012). However, so far models and laboratory studies cannot fully reproduce the observed mutually exclusive presence of specific isomers in certain star formation regions. Understanding the formation of the C2H4O2 isomers is an important step to verify the formation of yet more complex molecules that are necessary for life. In this talk, I will present our latest results obtained at the ion accelerator ATOMKI facility in Debrecen (Hungary) using the novel ultrahigh vacuum ICA end station. Following a systematic approach, we have exposed mixtures of CO:CH3OH (1:1, 1:2, 2:1) to 200 keV and 1 MeV H+ at 20 K. Ices are monitored by means of FTIR spectroscopy and results compared with those from the analogue irradiation of a series of pure species including CO, CO2, CH4, CH3OH, methyl formate, and acetic acid. Results will be discussed in light of upcoming JWST mission.
REFERENCES
Favre et al. HCOOCH3 as a probe of temperature and structure in Orion-KL. A&A 532, A32, 2011
Bockelee-Morvan et al. New molecules found in comet C/1995 O1 (Hale-Bopp). Investigating the link between cometary and interstellar material. A&A, v.353, p.1101-1114, 2000
Balucani et al. Formation of complex organic molecules in cold objects: the role of gas-phase reactions, Monthly Notices of the Royal Astronomical Society: Letters, Volume 449, Issue 1, 01, Pages L16–L20, 2015
El-Abd et al. Interstellar Glycolaldehyde, Methyl Formate, and Acetic Acid. I. A Bimodal Abundance Pattern in Star-forming Regions. ApJ, 883:129 (24pp), 2019
Gerakines et al. Ultraviolet processing of interstellar ice analogs: I. Pure ices. Astronomy and Astrophysics 312(1):289-305, 1996
Bennett and Kaiser. On the Formation of Glycolaldehyde (HCOCH2OH) and Methyl Formate (HCOOCH3) in Interstellar Ice Analogs. ApJ 661 899, 2007
Oberg et al. Formation rates of complex organics in UV irradiated CH_3OH-rich ices. I. Experiments. A&A, Volume 504, Issue 3, pp.891-913, 2009
Modica and Palumbo. Formation of methyl formate after cosmic ion irradiation of icy grain mantle. A&A 519, A22, 2010
de Barros et al. Cosmic ray impact on astrophysical ices: laboratory studies on heavy ion irradiation of methane. A&A 531, A160, 2011
Modica et al. Formation of methyl formate in comets by irradiation of methanol-bearing ices. Planetary and Space Science. 73(1):425–429, 2012
How to cite: Traspas Muiña, A., Ioppolo, S., Herczku, P., Juhász, Z., Kovács, S. T. S., Mifsud, D. V., Kaňuchová, Z., Mason, N., McCullough, R., and Sulik, B.: Formation and fate of methyl formate in space upon ion irradiation and its astrophysical relevance, Europlanet Science Congress 2021, online, 13–24 Sep 2021, EPSC2021-32, https://doi.org/10.5194/epsc2021-32, 2021.
Hexamethylentetramine has drawn a lot of attention due to its potential to produce prebiotic species. This work aims to gain a better understanding in the chemical processes concerning methylamine under astrophysically relevant conditions. In particular, this work deeps into the formation of N-heterocycles in interstellar ice analogs exposed to UV radiation, which may lead to the formation of prebiotic species.
Experimental simulations of interstellar ice analogs were carried out in ISAC. ISAC is an ultra-high vacuum chamber equipped with a cryostat, where gas and vapour species are frozen forming ice samples. Infrared and ultraviolet spectroscopy were used to monitor the solid phase, and quadrupole mass spectrometry served to measure the composition of the gas phase. The variety of species detected after UV irradiation of ices containing methylamine revealed the presence of 12 species which have been already detected in the ISM, being 4 of them typically classified as complex organic molecules: formamide (HCONH2), methyl cyanide (CH3CN), CH3NH and CH3CHNH. Warming up of the irradiated CH3NH2-bearing ice samples lead to the formation of trimethylentriamine (TMT), a N-heterocycle precursor of HMT, and the subsequent synthesis of HMT at temperatures above 230 K.
How to cite: Carrascosa, H., González Díaz, C., Muñoz Caro, G. M., C. Gómez, P., and Sanz, M. L.: Photon and thermal processing of CH3NH2-bearing ices. Synthesis of prebiotic species at low temperature (such as formamide, ethylamine, and methylcyanide), and N-heterocycles during warm up., Europlanet Science Congress 2021, online, 13–24 Sep 2021, EPSC2021-51, https://doi.org/10.5194/epsc2021-51, 2021.
Introduction: Millimeter and centimeter observations are discovering an increasing number of interstellar complex organic molecules (iCOMs) in a large variety of star-forming sites from the earliest stages of star formation to protoplanetary disks.
To correctly interpret the distribution and abundances of iCOMs observed along the formation process of a Sun-like star (i.e. in pre-stellar dense cores1,2, hot corinos around protostars3,4, in the associated jets and outflows5,6, and protoplanetary disks7), we need to first comprehend their formation processes and the mechanism responsible for their release in gas-phase. In this context, it is pivotal to understand the influence of the solid phase interactions between iCOMs and grain surface in the thermal desorption process and the subsequent presence of molecular species in the gas phase. Thermal desorption can be characterized through laboratory experiments using interstellar ice analogs deposited on grains similar to interstellar ones deriving important parameters such as the desorption temperatures and energies. Up to now, temperature-programmed desorption (TPD) experiments have been carried out mainly from graphite and amorphous water ice surfaces8,9,10,11. As far as we know, TPD experiments from grain surfaces are lacking in the literature, although mineral matrices can selectively adsorb, protect, and allow the iCOMs concentration on their surface. Molecules can diffuse inside the grains and so, the presence of grains can influence the desorption and release of the iCOMs in the gas phase.
Laboratory measurements: We report our new recently published laboratory results12 on TPD experiments of astrophysical relevant ice mixtures of water, acetonitrile (CH3CN), and acetaldehyde (CH3COH) from micrometric grains of silicate olivine ((Mg,Fe)2SiO4) used as interstellar dust analog on which the icy mixtures were condensed at 17 K inside the ultra-high vacuum (UHV) chamber (P~6.68 × 10-10 mbar). The ice mixtures condensed on olivine dust were heated at a constant rate, so the molecules diffused and desorbed from the dusty sample, and in situ TPD analysis was made.
Results: We found that in the presence of grains, only a fraction of acetaldehyde and acetonitrile desorbs at about 100 K and 120 K respectively, while 40% of the molecules are retained by fluffy grains of the order of 100 μm up to temperatures of 200 K. In contrast with the typical assumption that all molecules are desorbed in regions with temperatures higher than 100 K, this result implies that about 40% of the molecules can survive on the grains enabling the delivery of volatiles towards regions with temperatures as high as 200 K. This may be important in protoplanetary disks where the submicrometric interstellar grains begin to agglomerate into fluffy grains of hundreds of microns. The diffusion of molecules on the silicate surface is a valuable process enabling the permanence of the ices in the inner part of the disk. This implies that O-rich and N-rich volatiles ice can survive up to ∼ 200 K, broadening the snowlines of O- and N-bearing molecules, such as CH3CN and CH3COH. The presence of dust grains in the protoplanetary disk can therefore determine the approach of the snowlines towards the star and allow the presence of water and volatile species in Earth-like planets forming region.
These studies offer a necessary support to interpret observational data and may help our understanding of iCOMs formation providing an estimate of the fraction of molecules released at various temperatures.
- G. A. Blake, E. C. Sutton, C.R. Masson, T.G. Phillips, ApJ. 1987, 315
- E. Herbst & E. F. van Dishoeck, ARA&A. 2009, 47
- S. Cazaux, A. G. G. M. Tielens, C. Ceccarelli et al., ApJ. 2003, 593
- C. Ceccarelli, P. Caselli, E. Herbst, A. G. G. M. Tielens & E. Caux, Protostars and Planets V. 2007, 47
- R. Bachiller, & M. Pérez Gutiérrez, ApJ. 1997, 48
- C. Codella, B. Lefloch, C. Ceccarelli et al., A&A. 2010, 518
- J. Lee, S. Lee, G. Baek et al., Nature Astronomy. 2019, 3
- M. P. Collings, M. A. Anderson, R. Chen et al., MNRAS. 2004, 354
- T. Hama, N. Watanabe, A. Kouchi & M. Yokoyama, ApJL. 2011, 738
- J. Shi, G. A. Grieves & T. M. Orlando, ApJ. 2015, 804
- H. Chaabouni, S. Diana, T. Nguyen & F. Dulieu, A&A. 2018, 612
- M. A. Corazzi, J. R. Brucato, G. Poggiali, L. Podio, D. Fedele & C. Codella, accepted on ApJ. 2021
How to cite: Corazzi, M. A., Brucato, J. R., Fedele, D., Codella, C., Podio, L., and Poggiali, G.: The role of olivine grains on thermal desorption of astrophysical relevant ice mixtures of acetaldehyde and acetonitrile , Europlanet Science Congress 2021, online, 13–24 Sep 2021, EPSC2021-94, https://doi.org/10.5194/epsc2021-94, 2021.
Introduction
Radio Echo Sounding (RES) has been employed in planetary missions to survey the Moon, Mars, and Comet 67P/Churyumov-Gerasimenko (Porcello et al., 1974; Plaut et al., 2007; Kofman et al., 2015) and it will be applied, among others, in future investigations of the Galilean moons (JUICE mission - Grasset et al., 2013; EUROPA CLIPPER mission – Blankenship et al., 2018), and asteroids (e.g. AIDA mission - Herique et al., 2019). The ability of a radar signal to penetrate and image the subsurface layers depends on both the dielectric permittivity and magnetic permeability of the materials composing the crust. Thus, laboratory experiments are essential to improve our capability to estimate the radar performance and interpret the radar data collected in different scenarios. We present here the results of the electromagnetic measurements conducted for different porosities of a powdered L5 chondrite sample (a good simulant of the surface of Ganymede and S type asteroids), in the frequency range of interest for planetary radar sounders (1 MHz-1 GHz).
Methodology
The analyzed meteorite was classified as an ordinary L5 chondrite, with shock grade S2 and weathering W2 (Cosciotti et al.,2021). A fragment of the solid meteorite was pulverized in a jaw crusher and then sieved, to obtain five different granulometric samples, from <125 μm to 800 μm. The grain density of the powdered sample is ρg=(3.331±0.001)g/cm3, value that agrees with those found in literature for similar samples (Li et al., 2019). The electromagnetic measurements were carried out with a two port Vector Network Analyzer (VNA), employing a cage coaxial cell (Mattei et al., 2013). Sample porosity was progressively decreased by vibrating the sample inside the cell using a vibrating plate. The complex permittivity and permeability were estimated by the scattering parameter measurements and the Nicolson-Ross-Weir (NRW) algorithm (Nicolson and Ross, 1970). The real part of the effective permittivity of the two phases granular meteorite-air was studied using four different mixing formulas (table 1): Maxwell-Garnett, Bruggeman, Looyenga-Landau-Lifshitz (LLL) and Lichtenecker formula (Sihvola, 1999).
Table 1. εi and εe are respectively the permittivity of the environment and inclusions (i.e. air). f=1-Φ represents the volume fraction of the sample, where Φ is its porosity.
Results
Measurements with the VNA were performed on the granular chondrite sample at room temperature, for different porosities (27%-40%). For example, figure 1 and 2 illustrate the frequency spectra of the electromagnetic parameters for a porosity Φ=0.3. The plots also report the values of the air permittivity obtained with the empty cell, as they represent the instrument lower limit for an accurate measurement. Gray areas in the plots show values that cannot be considered reliable due to the NRW algorithm divergence (above 600 MHz) caused by the cell resonance or associated to the instrumental limits.
Figure 1. Complex permittivity frequency spectrum.
Note that, for the whole dataset, the real part of permittivity is fairly constant with frequency, whereas for frequencies lower than 10 MHz the imaginary part is below the limit of the instrument (figure 1). This result agrees with literature data as chondrites usually have low dielectric losses.
Moreover, our measurements show that the meteorite sample is slightly magnetic, with a Debye-like relaxation between 10 and 100 MHz (figure 2).
Figure 2. Complex permeability frequency spectrum.
Finally, the real part of permittivity at 100 MHz for all porosities was fitted with Lichtenecker formula (figure 3), to retrieve the value of solid permittivity (the value at zero porosity). We obtained εsol=9.2±0.2 , which is slightly lower than the value of the solid meteorite measured in Cosciotti et al., 2021.
Figure 3. of meteorite powder as a function of porosity fitted with Lichtenecker formula.
Conclusions
In this work we performed the electromagnetic characterization of crushed samples coming from an ordinary chondrite solid sample as a function of frequency and porosity. We found, in good agreement with literature data (e.g., Olhoeft and Strangway, 1974), that complex permittivity is strongly dependent on porosity. The presence of a Debye-like magnetic relaxation suggests that, not only dielectric but also magnetic loss should be accounted for when radar signal attenuation is estimated. In future laboratory work we will evaluate the effect of temperature, to better characterize the electromagnetic behavior of the samples.
Acknowledgements
This work has been supported by the Italian Space Agency through Contract ASI/INAF 2018-25-HH.0.
References
-Plaut, J. J., et al. (2007), Science, 316(5821), 92–95
- Porcello, L. et al., (1974), Proc. IEEE, 62(6), 769 – 783.
-Kofman, W. et al., (2015), Science 349, aab0639 1-6,
- Grasset, O. et al., (2013), Planetary and space science 78, 1–21.
- Blankenship, D. et al., (2018), 42nd COSPAR Scientific Assembly 42: B5-3.
- Herique, A. et al., (2019), EPSC, 2019, EPSC-DPS2019.
- Cosciotti, B. et al., (2021) Icarus (New York, N.Y. 1962) 362, 114426.
-Li, S. J. et al., (2019), Journal of Geophysical Research. Planets 124.11:2945-969.
- Mattei, E. et al., (2013), IEEE transactions on instrumentation and measurement 62.11, 2938–2942.
- Nicolson, A. M. and Ross, G. R. (1970), IEEE Trans. Instrum. Meas., 19, 4, 377–382.
- Sihvola A.H., (1999), IEE electromagnetic waves series. Institution of Electrical Engineer.
- Olhoeft, G. R. and Strangway, D. W., (1975), Earth and planetary science letters 24, 394–404.
How to cite: Brin, A., Lauro, S., Cosciotti, B., Mattei, E., and Pettinelli, E.: Laboratory investigations of the electromagnetic properties of a powdered L-chondrite, Europlanet Science Congress 2021, online, 13–24 Sep 2021, EPSC2021-213, https://doi.org/10.5194/epsc2021-213, 2021.
In this work we introduce a study of the North West Africa 12269 shergottite, recently discovered, using minimal and non-destructive analyses. A first chemical characterization will be performed by means of m-LIBS of an NWA 12269 sample. Micro-LIBS measurements of this and other Martian samples could be useful for supporting the interpretation of analyses coming from LIBS instruments onboard the rover Curiosity and Perseverance.
How to cite: Manzari, P., Tempesta, G., and Agrosì, G.: Spectroscopic study of North West Africa 12269, a recently classified Martian meteorite., Europlanet Science Congress 2021, online, 13–24 Sep 2021, EPSC2021-341, https://doi.org/10.5194/epsc2021-341, 2021.
Introduction: Laboratory measurements of extra-terrestrial materials like meteorites and ultimately materials from sample return missions can significantly enhance the scientific return of the global remote sensing data.
This motivated the addition of a dedicated Sample Analysis Laboratory (SAL) to complement the work of well established facilities like the Planetary Spectroscopy Laboratory (PSL) and the Astrobiology Laboratories within the Department of Planetary Laboratories at DLR, Berlin. SAL is being developed in preparation to receive samples from sample return missions such as JAXA Hayabusa 2 and MMX missions, the Chinese Chang-E 5 and 6 missions as well as the NASA Osiris-REX mission. SAL will be focusing on spectroscopic, geochemical, mineralogical analyses at microscopic level with the ultimate aim to derive information on the formation and evolution of planetary bodies and surfaces, search for traces of organic materials or even traces of extinct or extant life and presence of water.
Sample Analysis Laboratory: The near-term goalis to set up the facilities on time to receive samples from the Hayabusa 2 mission. The operations have already started in 2018 with the acquisition of a vis-IR-microscope and it will continue with the acquisition of: Field Emission Gun - scanning electron microscope (FEG-SEM), Field Emission Gun – electron microprobe analyser (FEG-EMPA), X-ray diffraction (XRD) system with interchangeable optics for μXRD analysis anda polarised light microscope for high resolution imaging and mapping
The facilities will be hosted in a clean room (ISO 5) equipped with glove boxes and micromanipulators to handle and prepare samples. All samples will be stored under dry nitrogen and can be transported between the instruments with dedicated shuttles in order to avoid them to enter in contact with the external environment. Based on current planning the first parts of SAL will be operational and ready for certification by end of 2022.
Current facilities: To characterize and analyse the returned samples, SAL facilities will work jointly with the existing spectroscopic capabilities of PLL.
PLL has the only spectroscopic infrastructure in the world with the capability to measure emissivity of powder materials, in air or in vacuum, from low to very high temperatures [1-3], over an extended spectral range from 0.2 to 200 µm. Emissivity measurements are complemented by reflectance and transmittance measurements produced simultaneously with the same set-up. Recently a vis-IR-microscope was added to extend spectral analysis to the sub-micron scale. In addition, the department is operating a Raman micro-spectrometer with a spot size on the sample in focus of <1.5 μm. The spectrometer is equipped with a cryostat serving as a planetary simulation chamber which permits simulation of environmental conditions on icy moons and planetary surfaces.
PLL leads MERTIS on BepiColombo as well as the BioSign exposure experiment on the ISS. The labs have performed laboratory measurements for nearly every planetary remote sensing mission. PLL has team members on instruments on the MarsExpress, VenusExpress, MESSENGER and JAXA Hayabusa 2 and MMX missions. Most recently we joined the Hayabusa 2 Initial Sample Analysis Team.The samples analyzed at PLL range from rocks, minerals, meteorites and Apollo and Luna lunar soil samples to biological samples (e.g. pigments, cell wall molecules, lichens, bacteria, archaea and other) and samples returned from the ISS (BIOMEX) [4, 5, 6] and the asteroid Itokawa (Hayabusa sample).
PLL is part of the “Distribute Planetary Simulation Facility” in European Union funded EuroPlanet Research Infrastructure (http://www.europlanet-2020-ri.eu/). Through this program (and its predecessor) over the last 9 years more than 80 external scientists have obtained time to use the PLL facilities. PLL has setup all necessary protocols to support visiting scientist, help with sample preparation, and archive the obtained data.
Outlook: DLR has started establishing a Sample Analysis Laboratory. Following the approach of a distributed European sample analysis and curation facility as discussed in the preliminary recommendations of EuroCares (http://www.euro-cares.eu/) the facility at DLR could be expanded to a curation facility. The timeline for this extension will be based on the planning of sample return missions. The details will depend on the nature of the returned samples. Moreover, SAL will be running in close cooperation with the Museum für Naturkunde in Berlin and it will be operated as a community facility (e.g. Europlanet), supporting the larger German and European sample analysis community.
References: [1] Ferrari et al., Am. Min., (2014), 99(4): p. 786-792. [2] Maturilli and Helbert, JARS (2014), 8(1): p. 084985. [3] A. Maturilli, et al., (2019) Infrared Remote Sensing and Instrumentation XXVII, 10.1117/12.2529266. [4] de Vera et al. (2012), PSS, 74(1): p. 103-110. [5] Serrano et al. (2014), PSS, 98: 191–197. [6] Serrano et al. (2015), FEMS Microbiology Ecology, 91(12): 2015, fiv126.
How to cite: Bonato, E., Schwinger, S., Maturilli, A., and Helbert, J.: A New Facility for the Planetary Science Community at DLR: the Planetary Sample Analysis Laboratory (SAL)., Europlanet Science Congress 2021, online, 13–24 Sep 2021, EPSC2021-561, https://doi.org/10.5194/epsc2021-561, 2021.
Please decide on your access
Please use the buttons below to download the presentation materials or to visit the external website where the presentation is linked. Regarding the external link, please note that Copernicus Meetings cannot accept any liability for the content and the website you will visit.
Forward to presentation link
You are going to open an external link to the presentation as indicated by the authors. Copernicus Meetings cannot accept any liability for the content and the website you will visit.
We are sorry, but presentations are only available for users who registered for the conference. Thank you.
Please decide on your access
Please use the buttons below to download the presentation materials or to visit the external website where the presentation is linked. Regarding the external link, please note that Copernicus Meetings cannot accept any liability for the content and the website you will visit.
Forward to session asset
You are going to open an external link to the asset as indicated by the session. Copernicus Meetings cannot accept any liability for the content and the website you will visit.
We are sorry, but presentations are only available for users who registered for the conference. Thank you.

