Can Raman spectroscopy determine the presence of ionic compounds? The use of water molecules as a indirect identification parameter
- University of the Basque Country, Faculty of Sciences and Technology, Department of Analytical Chemistry, Leioa, Spain (julene.aramendia@ehu.eus)
- Introduction
It is widely known that ionic compounds are hardly detected by Raman spectroscopy. Ionic bonds provide weak or no Raman signal under normal conditions but recent studies [1] have demonstrated that at high pressures and high temperatures quality Raman spectra can be obtained. Unfortunately, high pressures and temperatures change the structural composition of the sample to analyze. This is not the best option when the aim of the analysis is to characterize an unknown sample.
The characterization of ionic compounds such as chlorides is gaining strength in the space exploration field. For instance, in Mars surface phenomena linked to the past presence of water the characterization of chloride bearing deposits is crucial. The molecular characterization of these deposits could provide information about climate, water history and deposition dynamics [2, 3]. Moreover, in the study of terrestrial analogues it is also important in order to ascertain the capability of different compounds to preserve microorganisms, as a background science for Mars exploration.
Considering that Raman spectrometers were sent for the first time to the Mars surface onboard Perseverance Rover (MARS2020 mission), an effort to counter the weaknesses of this technique results worthy, even more, if it is focused on the resolution of key issues for Mars exploration.
In this way, in this work an indirect way to detect the presence of chlorides using the water molecules as a traceable Raman fingerprint is provided.
- Materials and methods
For the mentioned aim, a terrestrial brine sample was used as an analogue of a Mars chloride-bearing deposit. In addition, some hydrated chlorides standards such as MagCl2∙6H2O, CaCl2∙2H2O, FeCl3∙6H20 were also analyzed. In order to understand the contribution in the Raman signal of the major chloride in the sample, a standard of NaCl was also measured by Raman spectroscopy. Finally, a gypsum standard was considered for the work as it was the main Raman signal obtained in the analysis of the sample. For the analysis, a Renishaw InVia Raman confocal microscope was employed provided with several excitation lasers (532, 633 and 785 nm lasers) and a CCD detector. Its high spectral resolution (up to 1 cm-1) allowed obtaining high quality results. The measuring conditions were optimized at 20 seconds and 2 accumulations to obtain a good signal to noise ratio for the sample as well as for the analyzed standards in order to carry out fair comparisons.
- Results
As expected, Raman spectroscopic analyses performed on the brine samples did not provide any signal of the major compounds present in the sample which were mainly chlorides. Instead of that, the only detectable Raman signals were the bands for gypsum. The transparency of the major compounds allows the detection of a minor compound as it is the gypsum. However, it does not provide a complete characterization of the sample.
Considering this, an indirect identification through the presence of water molecules detected by the mentioned technique was tested.
First of all, the band area for the –OH region was measured for the sample Raman spectra. Then, as the gypsum was the only detected signal, a gypsum standard was measured in order to compare the –OH region area with the sample one. In this way, it was observed that the gypsum water molecules did not contribute enough to explain the area present in the sample spectrum. Therefore, by the comparison of the –OH region, a clear input of other hydrated phases to the –OH signals was observed in the sample.
Taking into account that NaCl was expected to be the main compound in the analyzed sample, analysis of a NaCl standard was also performed. In this way, it was confirmed the lack of Raman signal even on the –OH region as it was not hydrated. Thus, the lack of signals in the Raman region of the spectrum (different to that of gypsum), indicated that this extra –OH input belonged to other ionic hydrated species.
Several hydrated chloride standards were analyzed in order to evaluate their contribution in the –OH region. The contribution of the –OH band area of the analyzed compounds fit perfectly the –OH band area obtained for the sample. In this way, it was confirmed by Raman spectroscopy that other hydrated chlorides were present in the sample (Figure 1).
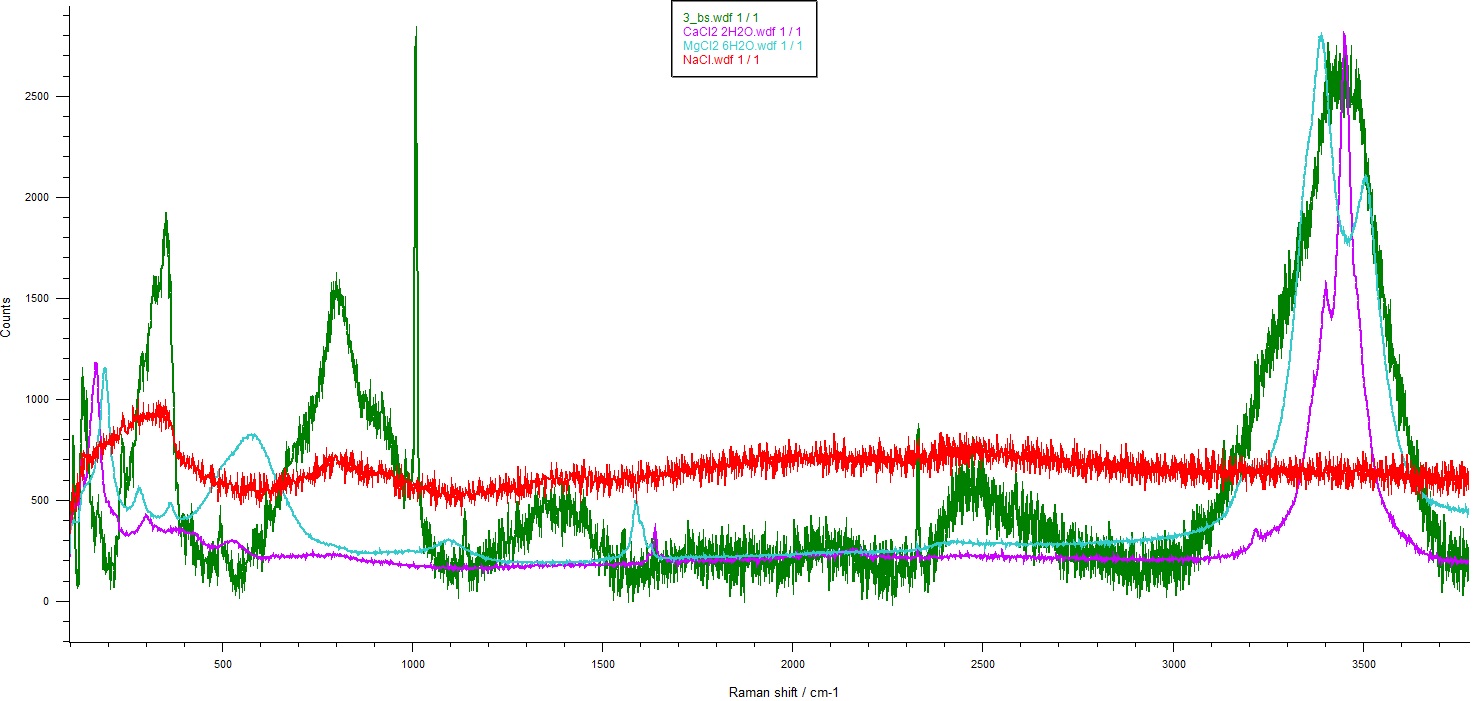
Figure 1. Raman spectra of a brine sample (3_bs) in green, NaCl in red, CaCl2 2H2O in purple and MgCl2 6H2O in cyan.
- Conclusions
Raman spectroscopy has been found as a successful technique to detect hydrated chlorides in brine samples. An accurate identification of the compounds was not possible yet but an indirect unambiguous methodology to detect their presence in a complicated sample is presented in this work. Raman spectroscopic analyses performed on hydrated chloride standards provide some broad and overlapping Raman bands. Nevertheless, the spectra obtained for the real sample did not present those bands. Therefore, the methodology presented on this work can be very useful.
- References
[1] Tian Y., Xiao W., He Y., Zhao H., Jiang F., Tan D., Chen M., Raman spectroscopy of sodium chloride under high-pressure and high-temperature. 2019, https://doi.org/10.48550/arXiv.1903.11824
[2] Leask E. K., Ehlmann B. L., Evidence for Deposition of Chloride on Mars From Small-Volume Surface Water Events Into the Late Hesperian-Early Amazonian. AGU Advances 3, e2021AV000534, 2022.
[3] Uriarte L. M., Dubessy J., Boulet P., Baonza V. G., Bihannic I., Robert P. Reference Raman spectra of synthesized CaCl2 ·nH2O solids (n=0, 2, 4, 6). Journal of Raman Spectroscopy 46, 822–828, 2015.
- Acknowledgements
This work has been funded by the “Raman On Mars” project (Grant No. PID2019-107442RB-C31), funded by the Spanish Agency for Research (MICINN and the European Regional Development Fund), and the “Study of Alteration Processes in Terrestrial and Planetary materials” strategic project (ref. PES 21/88), funded by the University of the Basque Country (UPV/EHU.
How to cite: Aramendia, J., Poblacion, I., Huidobro, J., Coloma, L., Garcia-Florentino, C., Arana, G., Castro, K., and Madariaga, J. M.: Can Raman spectroscopy determine the presence of ionic compounds? The use of water molecules as a indirect identification parameter, Europlanet Science Congress 2022, Granada, Spain, 18–23 Sep 2022, EPSC2022-1046, https://doi.org/10.5194/epsc2022-1046, 2022.

