Chemical composition of erupted brines on Europa
- 1NASA Jet Propulsion Laboratory, California Institute of Technology (elodie.lesage@jpl.nasa.gov)
- 2University of Maryland College Park
- 3NASA Goddard Space Flight Center
1. Introduction
Liquid water reservoirs in Europa's icy crust, if they exist, could represent the most accessible liquid water bodies in the outer solar system. Previous studies have demonstrated that freezing cryoreservoirs might trigger eruptions due to the pressurization associated with volume change as liquid water expands to become water ice [1, 2]. Locating potentially stored and erupted brines is key for the exploration of ocean worlds and the search for habitability and life beyond Earth.
Here, we aim to numerically model the coupled chemical evolution and pressurization of freezing brines stored in Europa’s ice shell using current best estimates of the oceanic composition [3] to predict the composition of erupted cryolava. This composition varies with time, as salts concentrate during freezing [4], which could lead to erupted brines of varying composition depending on the reservoir frozen fraction when the eruption is triggered. This could explain the variations of albedo observed around features potentially associated with cryovolcanism, for example smooth plains, as shown in Fig. 1.
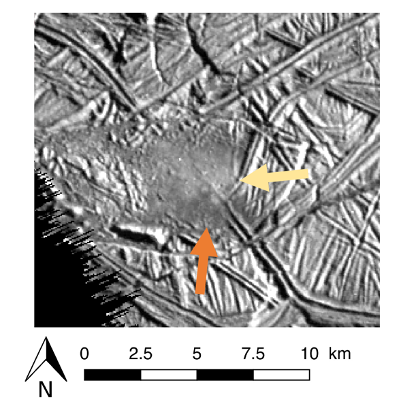
Figure 1: Smooth plain on Europa’s surface with morphology consistent with eruption of liquid cryolava [1]. The yellow and orange arrow indicate two areas of different albedo, which may have been emplaced by two eruptive events of brines of different composition. NASA Galileo image 9352r.
2. Methods
Cryomagma chemistry Whether they are formed by in-situ melting [5] or intrusion of oceanic water [6], the best estimate for cryomagmatic fluid composition is provided by models of evolution of Europa’s interior and ocean. An example cryomagma composition used in our model, consistent with oceanic compositions predicted by recent literature [3], in wt.%, is 99.0% H2O, 0.32% Na, 0.28% Cl, 0.16% bicarbonate, 0.21% sulfate, 0.027% K, 0.013% Ca, and 0.002% Mg. The equilibrium freezing of oceanic brines is modeled using the software PHREEQC [7] to obtain the liquid and solid fraction of each component of the aqueous solution as a function of the temperature. We use these data as an input of the model presented below.
Model principle Previous studies [1, 2] demonstrated that internal overpressure increases in potential freezing cryoreservoirs as cryomagma transitions to the less-dense solid phase. The critical freezing time required to break the reservoir and trigger an eruption is a function of the reservoir chemical and physical parameters (see example in Fig. 2 for a spherical 500 m radius reservoir located 2 km below the surface and filled with pure liquid water [1, 2]). The cryomagma chemical evolution during freezing will affect the solution and formed ice densities, and can thus make the critical freezing time different from what was calculated by previous studies. We thus need to model the cryomagma composition and internal overpressure as coupled variables. By doing so, we will be able to predict a realistic critical freezing time to trigger eruptions, as well as the
cryomagma composition when the eruption begins.
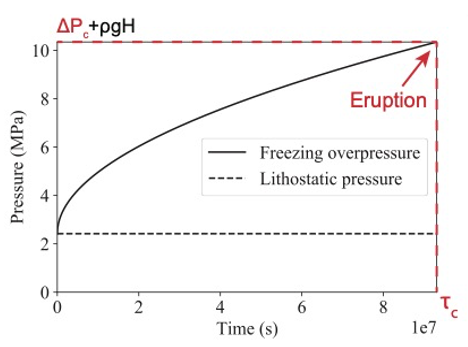
Figure 2: Pressure as a function of the time in a 500 m radius freezing cryoreservoir located 2 km below the surface. Here the cryomagma is composed of pure water only [2].
Numerical procedure We couple temperature-dependent compositional outputs from PHREEQC with a 1D numerical simulation using the finite differences method to simulate a freezing cryoreservoir. At each time step (i.e., reservoir temperature), we vary the composition of the formed ice and remaining aqueous solution. We modify several parameters accordingly, such as the liquid and solid densities and thermal conductivities. We ensure the energy and mass are conserved through time by calculating the cryomagma frozen fraction that balances the heat lost during each time step. As an output of the model, we can obtain the temporal evolution of: (i) the temperature in the reservoir, (ii) the composition of the formed solid and the remaining liquid and (iii) the internal overpressure. Finally, we can predict the composition of the erupted brines once (and if) the eruption is triggered.
Cyclic eruptions After the first eruption, cryomagma reservoirs may keep freezing and trigger several other eruptions as evoked in Lesage et al. [8]. We added the possibility to continue freezing and trigger several eruptions to our numerical simulation so that we can observe the evolution of erupted brines composition through time.
3. Preliminary results
Initial results demonstrate that salts concentrate in the reservoir during freezing, which decreases the solution freezing temperature and can make the freezing slower than expected with simpler models [1, 2]. On the other hand, the ice formed is initially mostly composed of pure liquid water and thus has a low density compared to the remaining liquid, which can accelerate the pressurization. The effect of both these mechanisms on the critical freezing time have to be quantified. The concentration of non-water components through time is also expected to result in cryolava flows with darker albedo and varying spectral signatures in the case of repeated eruptive events. These results will inform the upcoming missions JUICE (ESA) [9] and Europa Clipper (NASA) [10] on the spectral signatures that could indicate the presence of shallow sub-surface cryoreservoirs and provide information on their activity.
Acknowledgements
Portions of this research were carried out at the Jet Propulsion Laboratory, California Institute of Technology, under contract with the National Aeronautics and Space Administration (NASA). This work was supported by Scientific Exploration Subsurface Access Mechanism for Europa (SESAME) grant number 80NSSC19K0614 and by NASA’s Solar System Workings program (#80NSSC20K0139). The PHREEQC routine is available at https://github.com/MarcNeveu/frezchem.
References
[1] Lesage E. et al. (2020) Icarus, 335, 11336, [2] Lesage E. et al. (2022) The Planetary Science Journal, in press., [3] Melwani Daswani et al. (2021) Geophysical Research Letters, 48(18), [4] Zolotov M. Y. & Kargel J. S. (2009) Tucson, AZ: University of Arizona Press, [5] Kalousová et al. (2016) Journal of Geophysical Research: Planets, 121(12), 2444-2462, [6] Craft K. L. et al. (2016) Icarus, 274, 297-313, [7] Parkhurst, D. L. (1995). User's guide to PHREEQC, [8] Lesage et al. (2021) Icarus, 361, 114373, [9] Grasset O. et al. (2013) Planetary and Space Science, 78, 1-21, [10] Howell S. M. and Pappalardo R. T. (2020) Nature Communications, 11, 1311.
How to cite: Lesage, E., Howell, S. M., Naseem, M., Neveu, M., Melwani Daswani, M., and Vance, S. D.: Chemical composition of erupted brines on Europa, Europlanet Science Congress 2022, Granada, Spain, 18–23 Sep 2022, EPSC2022-301, https://doi.org/10.5194/epsc2022-301, 2022.

