Dichotomy of Cometary Outgassing
- 1Jet Propulsion Laboratory, California Institute of Technology, Science Division, Pasadena 91109, Califoria, United States of America (murthy.gudipati@jpl.nasa.gov)
- 2LISA, Université Paris-Est Créteil, CNRS, Université de Paris, IPSL, Créteil Cedex, France
- 3Physics Institute, Space Research and Planetary Sciences, University of Bern, Sidlerstrasse 5, CH-3012 Bern, Switzerland
Abstract
Understanding the composition of cometary nucleus will provide a direct link between present-day comets, KBOs, and Oort cloud objects and formation of cometesimals during the protoplanetary phase. We will understand whether cometary nucleus is primordial, and hence contain molecules of life. Our focus here is to derive compositional information of the nucleus of comet 67P-CG based on laboratory studies comparing with outgassing dichotomy of the comet, particularly that of CO2 and H2O and the corresponding minor volatiles.
Introduction
Interstellar ice grains and cometary outgassing have so much in common that it is hard to understand how such compositional similarity is preserved without the interstellar ice grains themselves preserved throughout the evolution of our solar system – from dense molecular clouds through protoplanetary disk to evolved solar system that has the cometary precursors – Kuiper Belt Objects and Oort Cloud.
While the link seems to be obvious, chemical evolution of ice grains in the protoplanetary phase is far from well understood. Particularly, if the ice particles were to cross the ice-line towards the protostar and sublimate leaving the refractory grains behind. It is hard to determine how similar composition is put together in the formation of cometary precursors and cometesimals. In addition to follow reaction pathways that could be used as tracers for the history of ice grain chemistry and transport, it is necessary to understand outgassing properties of long-period and short-period comets in order to correlate this outgassing to the physical and chemical composition of the nucleus through laboratory experiments and modeling. It is our goal to investigate and quantify the outgassing properties and dichotomy that is already noticed, but not yet fully understood.
Results
Here we present preliminary results from conventional temperature programmed desorption of ternary mixtures containing H2O, CO2, O2, and CO (Figure 1). While the first two are the major species resulting in mutually exclusive outgassing, of the last two super volatiles, O2 is predominantly correlated with H2O. Our laboratory data shows that excess CO2, O2, and CO that cannot be trapped into amorphous water ice, form separate ices at or below their individual condensation temperatures. Majority of this excess ice is sublimed at their respective pure ice sublimation temperatures. Some O2 and CO are trapped in the CO2 ice, which are seen to outgas along with CO2 sublimation. Keep in mind that in the mass spectrometer presented in Figure 1, contribution of CO due to fragmentation of CO2 is not removed. After completely outgassing aggregated ice other than H2O ice, amorphous to crystalline transition occurs around 120-140 K, during which almost all trapped volatiles (CO2, CO, and O2) within amorphous ice are released. The entrapment of these volatiles is also dependent on the starting ice composition. For example, when CO2 to H2O ratio is 1:3 vs. 1:1, more CO2 is trapped in H2O ice (Figure 1). However, we expect upper limit of CO2 trapping in H2O ice. When crystalline H2O ice sublimes, only a few percentage (< 3-5%) of the other volatiles are observed. Though the mixing ratio of our experiments is not exactly as it was observed by the Rosetta Mission, these data provide insight into the capacity of amorphous ice to trap other volatiles and sublimation temperatures of these volatiles forming pure ices. Our measurements are in qualitative agreement with earlier lab work (cf. Kouchi et al. 1994) though finer differences could be found, particularly around amorphous ice crystallization temperatures. Critical to the understanding of the ROSINA data from 67P-CG, is to use gas ratios in the lab comparable to the comet outgassing (Läuter et al. 2020) and vary the composition around this mixing ratio to determine the dependence of composition on sublimation properties. Keeping in mind that the ROSINA observed averaged outgassing of 67P-CG occurring from areas with very different illumination conditions, crystallization and sublimation seen in our experiments between 120 – 170 K seem to occur together in the observations. Future work includes further experimental work and comparison with observational data from Rosetta ROSINA instrument.
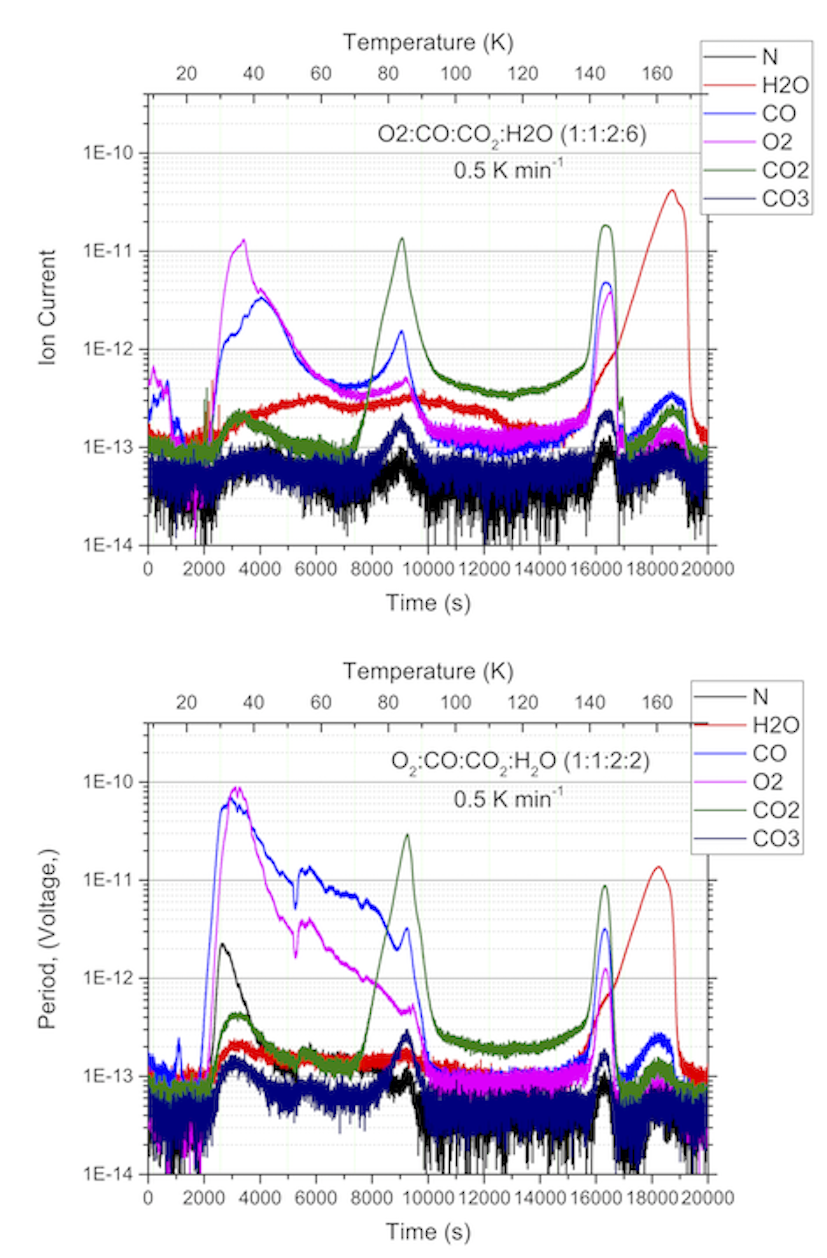
Figure 1: Laboratory data on outgassing properties of cometary ice analogs. It is evident that long-period comets originating from Oort cloud would have the significant outgassing of super volatiles, while short-period comets originating from KBOs and have gone through several orbits are depleted of these super volatiles, at least in the subsurface where the thermal equilibration could occur to temperatures beyond 80-100 K.
References
Kouchi, A., et al., A&A. 290, 1009-1018(1994)
Läuter, M., et al., MNRAS 498, 3995–4004 (2020)
Acknowledgements
This work was carried out at the Jet Propulsion Laboratory, California Institute of Technology, was under a contract with the National Aeronautics and Space Administration (NASA). Funding through NASA Discovery Data Analysis Program is gratefully acknowledged.
How to cite: Gudipati, M., Fleury, B., Rubin, M., and Altwegg, K.: Dichotomy of Cometary Outgassing, Europlanet Science Congress 2022, Granada, Spain, 18–23 Sep 2022, EPSC2022-334, https://doi.org/10.5194/epsc2022-334, 2022.

