Jarosite formation from olivine and rasvumite in Martian meteorite MIL 090030
- University of the Basque Country, Faculty of Science and Technology, Department of Analytical Chemistry, Leioa, Spain (juanmanuel.madariaga@ehu.eus)
- Introduction
Jarosite (KFe3(SO4)2(OH)6) was found by rover Opportunity in Martian surface at Meridiani Planum as well as by Spirit at Gusev crater. This mineral needs specific conditions to be formed, such as acid pH and oxidant environment1. In addition, this mineral has been found in meteorites, related to other compounds like olivine, goethite or gypsum.
There are different hypothesis for the formation of jarosite from olivine. Hereunder, two of them are exposed.
The first one described the jarosite formation in nakhlite meteorites, when the olivine transform to iddingsite. Iddingsite is a mixture formed by smectite clays, iron oxides and ferrihydrite2. Besides, some studies propose that jarosite forms part of iddingsite3.
The formation of jarosite from olivine in iddingsite phase can be explained by the dissolution of the latter. The minerals formed depend on pH and the ions present in the solution. The formation of jarosite can be explained by these reactions, considering an olivine with 1.8 proportion of Mg and 0.2 of Fe4 which is dissolved in acid conditions (reaction 1).
To form jarosite, the cation Fe2+ must be oxidized to Fe3+ (reaction 2). Finally, this Fe3+ can react with dissolved sulphate to form jarosite. Depending on the presence of other cations in the solution, different types of jarosite can be formed.
Other hypothesis5 proposed for the formation of jarosite can be made considering that in the history of Mars there were cold and dry climate periods. Moreover, in Mars there was also a high volcanic activity that formed sulphate-rich deposits. Considering this, Niles et al.5 suggested that olivine grains in Martian surface could form Fe-rich sulphates, as jarosite, if they were exposed to H2SO4 rich aerosols at temperatures around -40 °C (cryogenic conditions). The cations needed to form jarosite could be incorporated from different minerals, being olivine the most important source5.
In Martian nakhlite Miller Range 090030 (MIL 090030), jarosite has been found next to olivine and rasvumite (KFe2S3) recurrently. This fact, lead to the assumption that they were related. However, to this day, no explanation for the presence of jarosite together with rasvumite and olivine has been proposed. In this study, a hypothesis for an alternative formation of jarosite is made based on Raman image analyses carried out on the MIL 090030 Martian meteorite.
- Materials and method
A thick slide of the MIL 090030 meteorite was analyzed by means of Raman spectroscopy image. For this aim, an inVia confocal micro-Raman spectrometer, provided with 785 nm and 532 nm excitation lasers and CCD detector, was used coupled to HR StreamLine Imager.
- Results and discussion
After detecting all minerals around olivine grains in the bulk of the meteorite, the distribution of the different mineral phases can be obtained using Raman image. In this way, Figure 1 shows the mineral distribution of a rasvumite/jarosite rich region in MIL 090030. Moreover, it can be appreciated how jarosite and rasvumite are surrounded by magnetite and cristobalite.
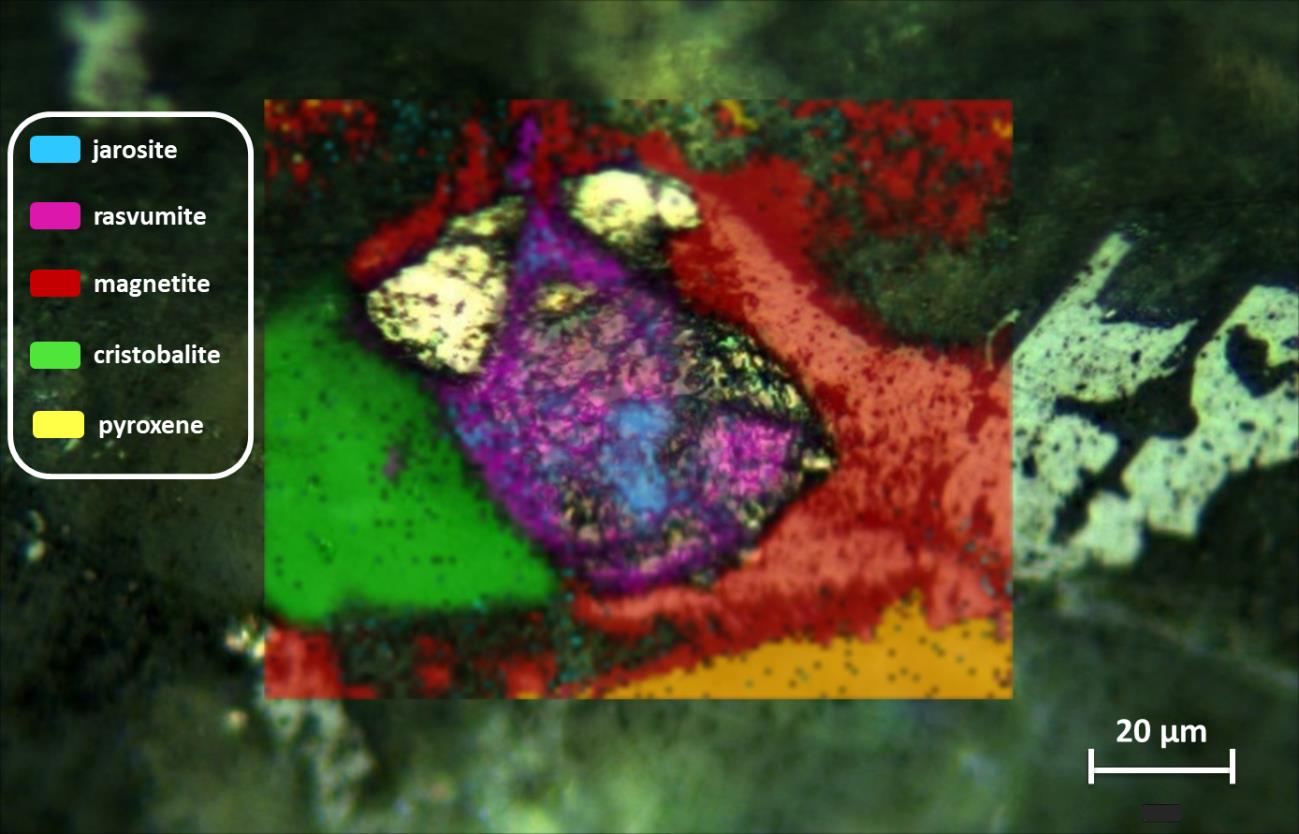
Figure 1. Raman image performed on the MIL090030 meteorite in a rasvumite/jarosite rich region.
Based on the findings detected in the analysed MIL 090030 fragment, the formation of jarosite from olivine and rasvumite is proposed following these four reactions:
On the one hand, rasvumite could react with H2O and O2 from Martian atmosphere to form magnetite (Fe3O4) (reaction 4). The soluble ions formed can arrive to other zones of the Martian surface travelling through cracks and pores.
The protons formed in reaction 4 can dissolve partially the olivine giving rise to an olivine richer in forsterite (reaction 5). The SiO2 formed can be dissolved in water to form H2SiO3 or H4SiO4. This solution can then precipitate as quartz when water evaporates.
On the other hand, rasvumite can react also directly with H2O and O2 to form jarosite and free sulphate anion (reaction 6). Sulphate anions formed in reactions 4 and 6, can interact with iron cations from reaction 5 and potassium cations from reaction 4 to form jarosite (reaction 7).
The high pressures and temperatures reached during the impacts suffered by the meteorite can alter some minerals present in the meteorite. In the MIL 090030 meteorite, quartz was not detected. Instead of this mineral phase, its high temperature and pressure polymorphs (cristobalite, coesite and stishovite) were detected in the rasvumite/jarosite rich region. The presence of these polymorphs indicates that the reactions 1-4 occurred in Mars.
- Conclusions
According to the mineral distribution in MIL 090030, based on Raman image results, an explanation for the formation of jarosite from olivine and rasvumite is proposed. This formation is related to reactions of rasvumite with Martian atmosphere compounds and dissolution of olivine from the Martian surface.
- Acknowledgments:
Authors are grateful to NASA for accessing to the MIL 090030 Martian meteorite sample through the loan agreement between NASA's JSC and the UPV/EHU. This work has been funded by the “Raman On Mars” project (Grant No. PID2019-107442RB-C31), funded by the Spanish Agency for Research (MICINN and the European Regional Development Fund), and the “Study of Alteration Processes in Terrestrial and Planetary materials” strategic project (ref. PES 21/88), funded by the University of the Basque Country (UPV/EHU.
- Bibliography:
1) Papike, J.J., Karner, J.M., Shearer, C.K.: Comparative planetary mineralogy: implications of martian and terrestrial jarosite. A crystal chemical perspective, Geochim. Cosmochim. Acta., Vol. 70, pp. 1309-1321, 2006.
2) Swindle, T.D., Treiman, A.H., Lindstrom, D.J., Burkland, M.K., Cohen, B.A., Grier, J.A., Li, A., Olson, E.K.: Noble gases in iddingsite from the Lafayette meteorite: evidence for liquid water on Mars in the last few hundred million years. Meteorit. Planet. Sci., Vol. 35, pp. 107-115, 2000.
3) Noguchi, T., Nakamura, T., Mizawa, K., Imae, N., Aoki, T.,Toh, S.: Laihunite and jarosite in the Yamato 00 nakhlites: Alteration products on Mars? J. Geophys. Res., Vol. 114, 2009.
4) King, H.E., Plümper, O.,Geisler, T.,Putnis, A.: Experimental investigations into the silicification of olivine: Implications for the reaction mechanism and acid neutralization. Am. Min., Vol. 96, pp. 1503-1511, 2011.
5) Niles, P.B., Michalski, J., Ming, D.W., and Golden, D.C. Elevated olivine weathering rates and sulphate formation at cryogenic temperatures on Mars. Nat. Commun., Vol. 8, 2017.
How to cite: Madariaga, J. M., Coloma, L., Aramendia, J., Huidobro, J., Poblacion, I., Garcia-Florentino, C., Castro, K., and Arana, G.: Jarosite formation from olivine and rasvumite in Martian meteorite MIL 090030, Europlanet Science Congress 2022, Granada, Spain, 18–23 Sep 2022, EPSC2022-518, https://doi.org/10.5194/epsc2022-518, 2022.

