Development of Surface Enhanced Raman Spectroscopy Substrates for Detection of Trace Biomarkers
- University of Southampton, United Kingdom of Great Britain – England, Scotland, Wales (dlb1e23@soton.ac.uk)
The use of Raman spectroscopy in planetary science has seen recent success with the deployment of the SHERLOC [1] and SuperCam [2] instruments on the Mars 2020 mission, and the development of instruments such as the ExoMars Raman Laser Spectrometer [3]. Raman spectroscopy is a useful technique for distinguishing mineral species and has the potential to identify organic chemicals, contributing to the search for extraterrestrial biomarkers. However, the limit of detection for the current set of Raman spectroscopy instruments on planetary missions may prevent the detection of trace concentrations of organic material present in the surface regolith or ice [4].
Fig. 1: Nanofabrication process flow for the creation of quartz nanopillars.
One method to improve the detection limit of Raman spectroscopy is to employ surface enhanced Raman spectroscopy (SERS). This technique exploits the interaction between incoming laser radiation, the analyte molecule, and hotspot regions associated with nanometer scale gaps in highly conductive materials such as silver and gold [5, 6]. Silver substrates typically provide high SERS enhancement factors [7], but suffer from degradation under storage conditions, with the enhancement factor reducing in a matter of days. Silver chloride provides one possible solution to this issue; silver chloride can be activated using the same laser wavelength as used for Raman spectroscopy (e.g. 532 nm), which creates regions of metallic silver which then contribute to SERS enhancement [8, 9].
We created geometrically ordered substrates using a nanofabrication process flow (fig. 1). Quartz glass wafers were patterned using electron beam lithography and then etched to provide an ordered pattern of nanopillars with diameter ~150 nm. These pillars were then coated with silver chloride via repeated immersion in solutions of silver nitrate (AgNO3) and sodium chloride (NaCl).
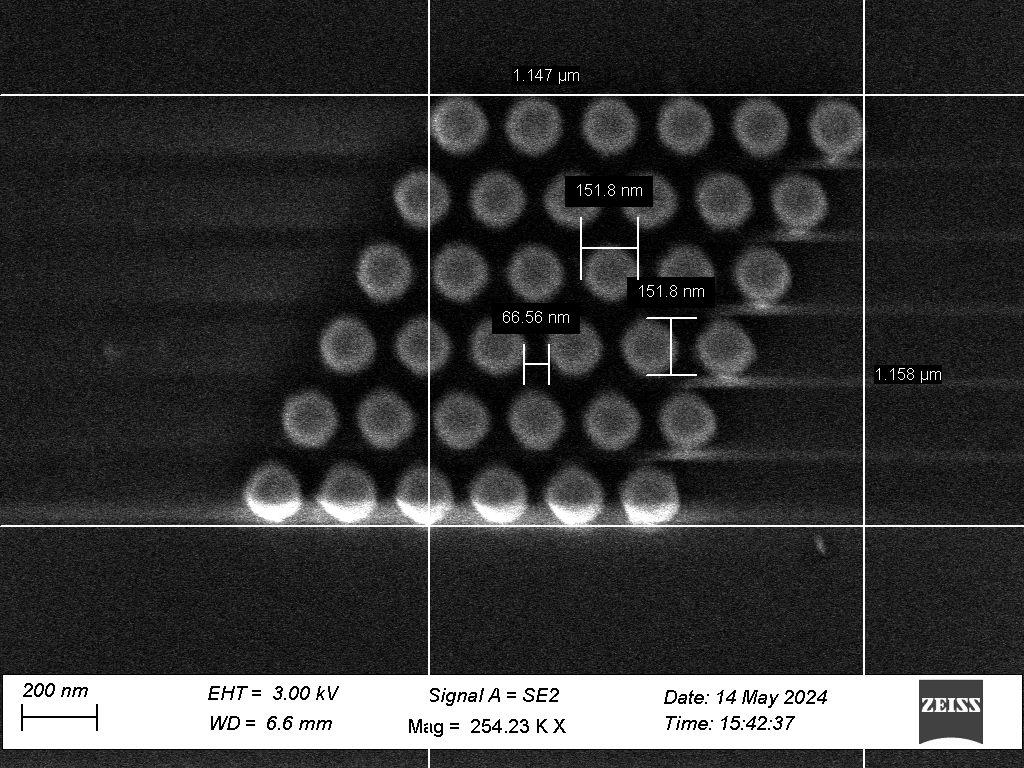
Fig. 2 SEM scan of hexagonally packed nanopillars, with geometric characterisation measurements
We present characterisation of the production process, using high aspect ratio nanofabrication techniques. Scanning electron microscopy (Fig 2, Fig 3) was used to characterise the geometry of the substrates. In addition, glass microscope slides which had not been altered with nanofabrication techniques were also coated with silver chloride, in order to evaluate the effect of the surface geometry.
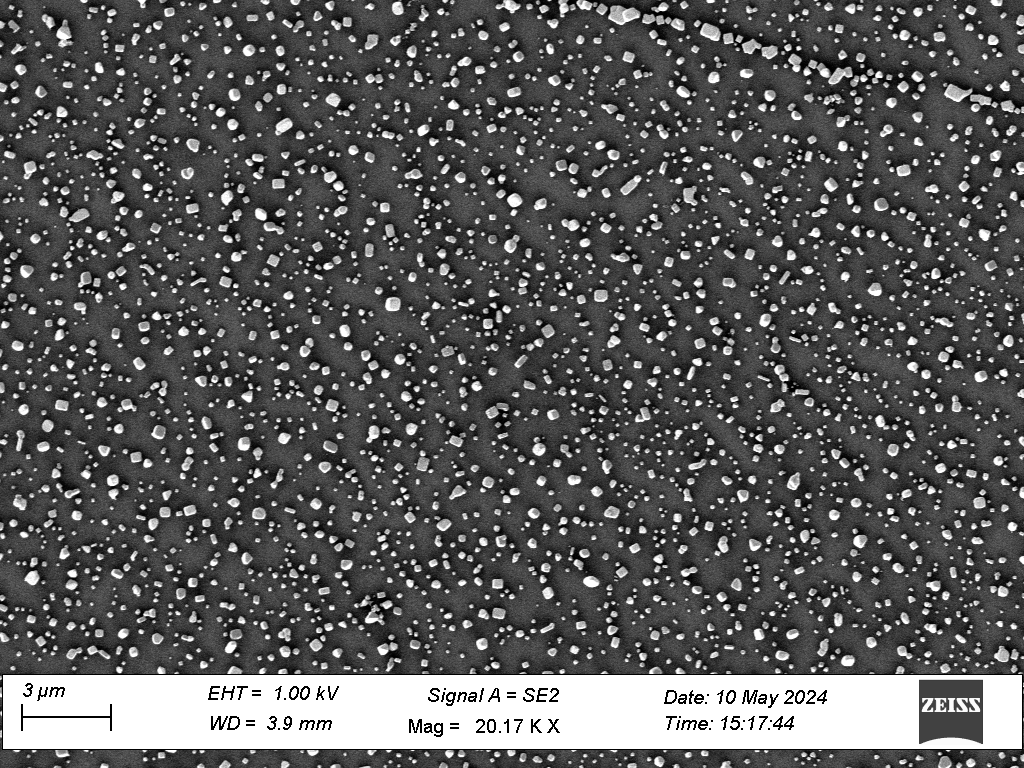
Fig. 3 SEM scan of silver chloride crystals on a glass slide
We also present validation and analysis of the SERS enhancement effect from these substrates. Glycine, β-carotene and L-histidine were used as probe biomarker molecules. Raman spectra were taken using a 532nm benchtop spectrometer. Control measurements were performed using the same biomarkers on uncoated glass microscope slides.
[1] Bhartia, R., et al., Perseverance’s Scanning Habitable Environments with Raman and Luminescence for Organics and Chemicals (SHERLOC) Investigation. Space Science Reviews, 2021. 217(4): p. 58.
[2] Maurice, S., et al., The SuperCam instrument suite on the Mars 2020 rover: science objectives and mast-unit description. Space Science Reviews, 2021. 217: p. 1-108.
[3] Rull, F., et al., The Raman laser spectrometer for the ExoMars rover mission to Mars. Astrobiology, 2017. 17(6-7): p. 627-654.
[4] McKay, C.P., et al., The Icebreaker Life Mission to Mars: A Search for Biomolecular Evidence for Life. Astrobiology, 2013. 13(4): p. 334-353.
[5] Le Ru, E.C., et al., Surface Enhanced Raman Scattering Enhancement Factors: A Comprehensive Study. The Journal of Physical Chemistry C, 2007. 111(37): p. 13794-13803.
[6] Lee, S.J., et al., Surface-enhanced Raman spectroscopy and nanogeometry: The plasmonic origin of SERS. The Journal of Physical Chemistry C, 2007. 111(49): p. 17985-17988.
[7] Sharma, B., et al., SERS: Materials, applications, and the future. Materials Today, 2012. 15(1): p. 16-25.
[8] Volkan, M., D.L. Stokes, and T. Vo-Dinh, A sol–gel derived AgCl photochromic coating on glass for SERS chemical sensor application. Sensors and Actuators B: Chemical, 2005. 106(2): p. 660-667.
[9] Matikainen, A., et al., A solution to the fabrication and tarnishing problems of surface-enhanced Raman spectroscopy (SERS) fiber probes. Scientific Reports, 2015. 5(1): p. 8320.
How to cite: Bowden, D. and Sykulska-Lawrence, H.: Development of Surface Enhanced Raman Spectroscopy Substrates for Detection of Trace Biomarkers, Europlanet Science Congress 2024, Berlin, Germany, 8–13 Sep 2024, EPSC2024-1024, https://doi.org/10.5194/epsc2024-1024, 2024.