- 1Czech Academy of Sciences, Institute of Geophysics, Prague 4, Czechia (kryza@ig.cas.cz)
- 2School of Physical Science, STEM, The Open University, Milton Keynes, United Kingdom
- 3Institute of Chemical Process Fundamentals of the CAS, v.v.i., Rozvojová 135/1, 16502 Prague 6, Czech Republic
- 4CNRS UMR-6112 LPG Nantes, France
- 5Centre for Earth Evolution and Dynamics (CEED), University of Oslo, Norway
- 6Institute of Planetary Research, DLR, Berlin, Germany
- 7Space Science and Technology Department, STFC Rutherford Appleton Laboratory, Oxford, UK
Introduction
Mud volcanism is a widely distributed geological phenomenon on Earth1. Likewise, it has been suggested that mud volcanoes might exist on some other solid-surface bodies in the Solar System, such as Mars2 and the dwarf planet, Ceres3. Since this phenomenon requires liquid water, extraterrestrial mud-volcano-like (MVL) structures represent key targets for studying the hydrology and potential habitability of subsurface environments on other planetary bodies. Thus, identifying the potential morphological signatures of these landforms beyond Earth is an important step in understanding the nature of aqueous environments within the Solar System. Previously performed low-pressure experiments have shown that in case of “cold” surfaces (-15 °C), low viscosity mud propagates similarly to pahoehoe lava types on Earth4, while in case of “warm” (+20 °C) and unconsolidated surfaces, mud “levitates'' and hence can be transported to longer distances5. The effect of composition, however, was not further tested nor discussed. We hypothesize that the potential muds on Mars or other planetary bodies may naturally contain a certain amount of salts which can affect their antifreezing and rheological properties. Therefore we address the question how the salt component in muds may affect their propagation over cold surface in reduced atmospheric pressures, namely those valid for recent Mars.
Methods
To test our hypothesis, we carried out 54 experiments by experimental procedure adapting the settings (Fig. 1) established for mud flow experiments performed in the Large Mars Vacuum Chamber at the Open University, UK by4,5. During the experiments, portions of mud were released onto a pre-cooled sand surface when the desired pressure (5.9±1 mbar) was reached. The mud was composed of D.I. water, bentonite and with various concentrations (0.5-10%) of salts, namely NaCl, MgSO4 (epsomite), Na2SO4 and CaSO4 were tested. Experiment progress was recorded by cameras situated at the top and sides of the sandbox and temperatures of sand and mud reservoir were measured by thermocouples. In complementary methods, we investigated the pressure-drop-induced evaporative cooling of the brine component on isolated samples in low pressure. Further, we calculated theoretical p-T paths by thermodynamic modeling and measured rheological properties of salty muds for reference Earth atmospheric pressure.
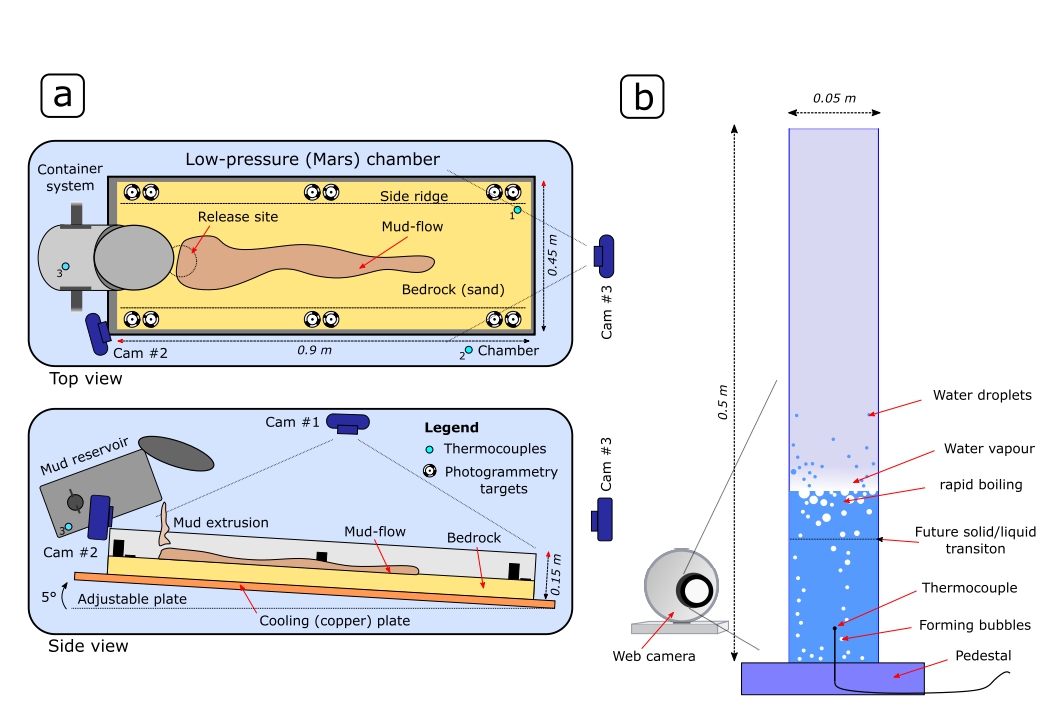
Fig. 1: Setup of the (a) flow and (b) tube experiments.
Results and discussion
Our experiments confirmed expected contrasting behavior of salty mud in decreased pressure (Fig. 2). Individual flows are characterized by unique spatial dispersion and morphological patterns for mutually comparative salt content. Results also revealed thresholds when different muds produce similar patterns and spreading style for highly different concentrations. Performed brine evaporative-cooling experiments showed that the maximum antifreeze potential has NaCl and therefore solutions with this salt are capable of sustaining their liquid state in much smaller pressures than those with other tested salts. The rheological measurements, on the other hand, revealed a contrasting impact of salt addition to viscosity drop of mud samples until these are supersaturated by salt (typically >5-10% concentration in dependence on salt type) and viscosity is further increased. Due to the synergistic effect of decreased viscosity (Fig. 3a) and anti-freezing effect (Fig. 3b) flows are spatially longest for various salts and, not necessarily directly proportional, to their concentrations. For example, 2.5% NaCl mud has a higher anti-freezing effect and lower viscosity compared to 10% MgSO4 (epsomite), resulting in long and narrow flows (Fig. 3c). The increased viscosity of MgSO4 effectively slows the flow, supports the formation of a protective crust and the development of serial, long lobes that maintain liquid mud in their interiors (Fig. 2b,3c). Both salts then exhibit similar spatial dispersion but entirely different styles of propagation and surface geometric pattern. These results are contrasting to previously published experiments and reveal that the increased content of salts leads to different regimes of mud propagation, not necessarily similar to pahoehoe lavas.

Fig. 2: Plan view of representative mud flows based on various salts and their concentrations. Visible are distinct patterns of individual flows and contrasting spatial dispersion.

Fig. 3 Interpretation of the rheological and thermodynamical effects on mobility of the mud mixtures. (a) shows the progressive increase and decrease of the mud bulk shear velocity in dependence on salt content. (b) is the interpretation of how the individual salts in various concentrations (sorted from lowest to highest effect) impact the freezing delay during the pressure-temperature change. (C) three main contrasting evolutionary trends in dependence on salt type and concentration: 1. multiple short flows that form as parallel lobes, 2. single long-narrow flow - that forms as serial rope-shaped lobes for 10% MgSO4 and lobe-less gently roped pattern for 2.5 NaCl, and 3. single wide and longer "sheet-shaped" flow. The most effective propagation is observed with a combination of 10% MgSO4 and 2.5% NaCl, where the synergistic effects of minimum viscosity and lowered freezing point are most pronounced.
All these findings therefore suggest that the salt type and concentrations dissolved within the muddy mixture are important factors in controlling the ultimate shapes, textures and dynamics of mud flows emplaced e.g. on Mars or on other bodies with a thin or non-existent atmosphere. Higher salinity levels extend the unfrozen state and promote the wider spatial dispersion of muds until they reach a certain point of saturation with salts. All this suggests that on Mars, contrary to Earth, salts can play an important role in shaping MVL structures. Variations in salt types and concentrations might help to, at least partly, explain the large variability in the shapes of these hypothesized martian MVL structures.
References
[1] Mazzini & Etiope (2017). Earth-Science Reviews, 168, 81-112; [2] Brož et al. (2023). Earth Surface Dynamics, 11(4), 633-661; [3] Ruesch et al. (2019). Nature Geoscience, 12(7), 505-509; [4] Brož et al. (2020). Nature Geoscience, 13(6), 403–407; [5] Brož et al. (2020). EPSL, 545.
How to cite: Krýza, O., Brož, P., Fox-Powell, M., Pěnkavová, V., Conway, S., Mazzini, A., Hauber, E., Sylvest, M., and Patel, M.: The effect of salts on the mobility and shapes of mudflows in low pressure environments, Europlanet Science Congress 2024, Berlin, Germany, 8–13 Sep 2024, EPSC2024-1134, https://doi.org/10.5194/epsc2024-1134, 2024.