Investigation of Hydrogen Clathrate Hydrates as Energy Storage Media for Planetary Installations
- 1University "G. d'Annunzio" of Chieti-Pescara, Pharmacy, Italy (pietro.diprofio@unich.it)
- 2University "A. Moro" of Bari, Chemistry, Italy (savino.longo@uniba.it)
- 3National Institute of Astrophysics (INAF), Catania, Italy (riccardo.urso@inaf.it)
The present communication is based on preliminary results within the PRIN 2022 PNRR project entitled “Low-cost, high-safety hydrogen storage into chemically-enhanced clathrate hydrates for energy storage in planetary infrastructures” (Brave New Worlds, CUP D53D2301693000, funded by the European Union – Next Generation EU) led by the University “G. d’Annunzio” of Chieti – Pescara, with the collaboration of University “Aldo Moro” of Bari, and the National Institute for Astrophysics of Catania (INAF) as research units. The aim of the project “Brave New Worlds” is to develop low-cost, high-safety media for the storage of hydrogen, which also have minimal technologic and maintenance requirements. Target storage media will be ideally suitable for space-based infrastructures on near planets (e.g., Mars) or satellites (e.g., Moon), where hydrogen will be produced from planetary water bodies by solar cell-powered electrolysis.
Currently, hydrogen is stored in pressurized cylinders, metal hydrides and similar compounds that require high energy consumption to store and recover H2, or in liquefied form. None of those storage technologies are suitable for planetary infrastructures, because of the high spacecraft payloads needed to carry cylinders, compressors, metallic media and other highly technological devices to be deployed and assembled in situ. Furthermore, the control of the compression of hydrogen into cylinders, or the temperature cycles for the sorption onto metal hydrides, or for liquefaction, require sophisticated, failure-prone control appliances.
The storage media developed in the present work are clathrate hydrates of hydrogen, a class of supramolecular solids consisting of water molecules organized in cage structures that can host one or more gas molecules. These systems represent a safer, technologically simpler, and cheaper alternative for large-scale hydrogen storage than traditional storage methods. Clathrate hydrates are usually formed under conditions of pressures around 5-10 MPa and temperatures of around 250-280 K, or, importantly for the present case, under lower pressures and very low temperatures.
Thus, important features that we are exploiting are the following:
- clathrate hydrates are essentially made up of water, an economical, ecological and safe compound par excellence. Having a potentially infinite life cycle, water is an ideal material for this purpose.
- Water is found on (or just below) planet and satellite surfaces.
- Sun-shaded or deep crater areas of planets and satellites reach temperatures as low as 30 K
- Hydrogen hydrates can form at very low temperatures under mild gas pressures
In the present work, we show how to overcome some critical points of hydrogen storage in clathrates, namely (i) slow capture kinetics, and (ii) low gravimetric content. As for point (i), here we report processes and molecules for improving the kinetics of the process of 1-2 orders of magnitude. The increase of the gravimetric content (point (ii)) has been addressed with the design and test of stabilizers (co-formers) of the hydrate cages, through a combination of rational design, quantum mechanical and molecular dynamics approaches, stochastic methods, and chemical synthesis. Preliminary results reported in the present contribution are: (i) the thermodynamic equilibrium curves of binary hydrogen hydrates in presence of stabilizers (tetrahydrofuran, cyclopentane; Fig. 1); (ii) the kinetics of formation of hydrogen hydrates under water-in-oil or oil-in-water emulsion systems (Fig. 2); and (iii) Raman spectroscopy data showing the presence of hydrogen within the small hydrate cages (512 dodecahedra) and possibly also within the large cages (51262 polyhedra) of sII crystal structures (Fig. 3).
The final goal will be to develop a hydrogen storage medium with a gravimetric H2 content around 4 wt%, which is demonstrably competitive with current top technologies at a fraction of the technological level and economic cost.
Fig. 1: P/T curve for THF/CP/hydrogen hydrate formation under different pressures
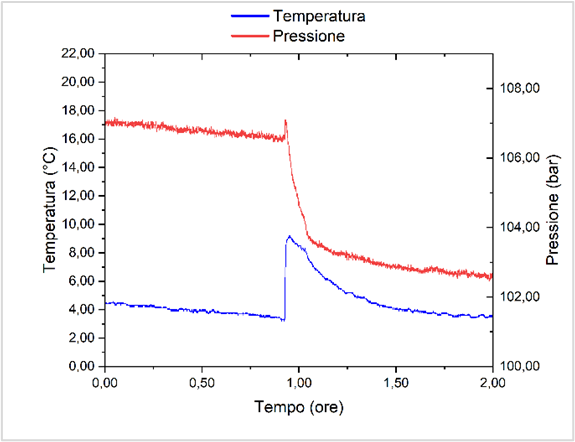
Figure 2: Kinetic P/T curves for the formation of THF/CP/H2 hydrate
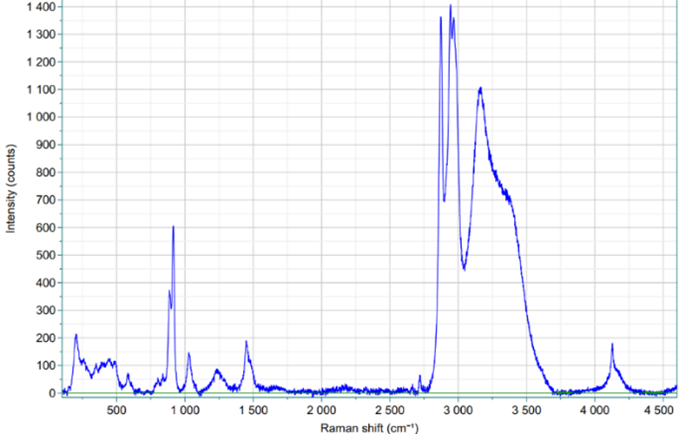
Figure 3: Raman spectrum of THF/CP/H2 hydrate at 70 bar
Bibliography
[1] Di Profio P, Arca S, Rossi F, Filipponi M. Int J Hydrogen Energy 2009;34:9173–80.
[2] Di Profio P, Arca S, Germani R, Savelli G. J Fuel Cell Sci Technol 2007;4:49–55.
[3] Di Profio P, Canale V, Germani R, Arca S, Fontana A. J Colloid Interface Sci 2018;516:224–31.
How to cite: Di Profio, P., Ciulla, M., Siani, G., Barbacane, N., Wolicki, R. D., Di Giacomo, S., Re, N., Marrone, A., Paciotti, R., Longo, S., Urso, R., Scire Scappuzzo, C., Palumbo, M. E., and Baratta, G.: Investigation of Hydrogen Clathrate Hydrates as Energy Storage Media for Planetary Installations, Europlanet Science Congress 2024, Berlin, Germany, 8–13 Sep 2024, EPSC2024-1169, https://doi.org/10.5194/epsc2024-1169, 2024.