The evaporation of volatile elements from metal melts: implications for volatile element depletions in metal-rich planetesimals
- 1Delft University of Technology, Faculty of Aerospace Engineering, Netherlands (e.s.steenstra@tudelft.nl)
- 2University of Münster, Institute of Mineralogy, Münster, Germany
- 3Max Planck Institute for Solar System Research, Göttingen, Germany
Introduction: The chemical composition of magmatic iron meteorites provides fundamental insights into planetary accretion processes. They are distinguished based on their trace element compositions and could represent the cores of more than 50 parent bodies [1]. The primary difference between the different groups is the degree of volatile element depletions, which increases from class I to IV iron meteorites [2]. For example, Cu, Ge and Ag concentrations of magmatic iron meteorites deviate up to 4 log units between the different magmatic iron meteorite groups [2]. The volatile element loss could have occurred prior to (i.e. nebular) or during parent body accretion and differentiation, for example during exposure of a liquid core following a catastrophic impact [3]. Investigating the mechanisms of volatile loss from requires experimental constraints on their volatility, for example during evaporation from metallic melts [3]. We previously determined the volatility of Cu, Ge, Ag and S [4]. Here, we extend the latter work to include most other volatile elements (Zn, Ga, Se, Cd, In, Sn, Sb, Te, Pb, Bi), and experimentally determined their evaporation from metallic melts as a function of pressure (10-4 to 1 bar), temperature (1573−1773 K) and time (5−120 min) for two end-member compositions (Fe versus FeS).
Approach: Evaporation experiments were performed in a high-temperature (vacuum) furnace. Experimental starting compositions consisted of metallic Fe or FeS starting powders doped with the (trace) elements of interest. After the required run time experimental run products were quenched in water, mounted in epoxy resin, polished and prepared for subsequent analyses by electron microprobe and LA-ICP-MS at the University of Münster. The LA-ICP-MS analyses were calibrated using the NIST 610 glass and 56Fe as an internal standard. A spot size of preferably 130 μm was used for virtually all analyses. To obtain reference concentrations of the starting materials prior to degassing, starting materials were synthesized at high P-T conditions in a piston cylinder press. The measured concentrations in the experimental run products were then normalized relative to the elemental concentrations measured in the undegassed, high P-T synthesized metal and sulfide liquids. Note that this approach also rules out any potentially significant matrix effects on LA-ICP-MS derived concentrations of degassed samples: both materials are (near)-identical in major element compositions [4].
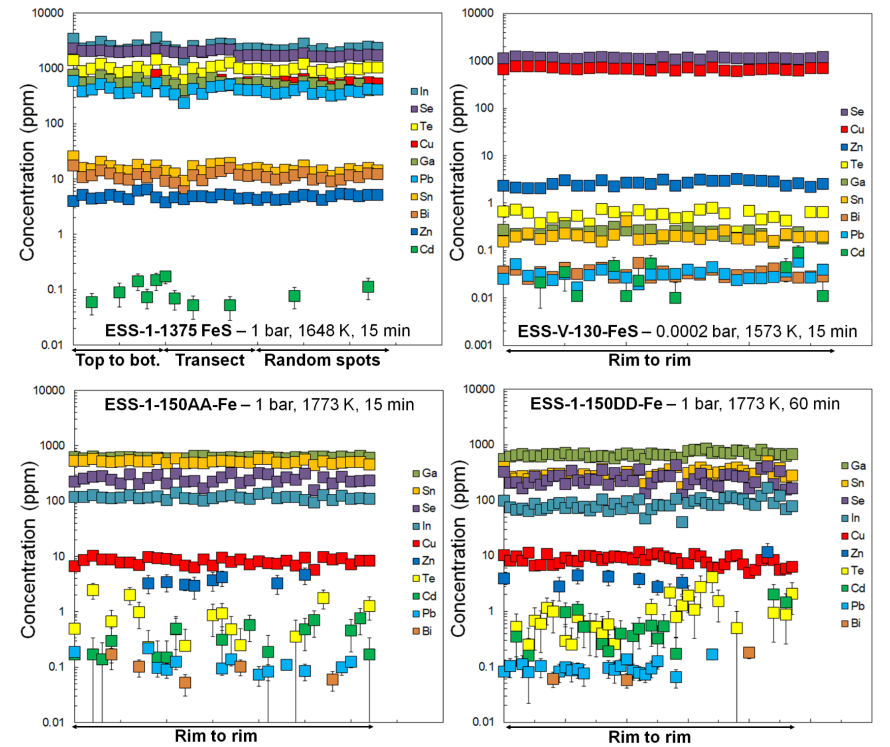
Results and discussion: Fig. 1 shows a typical experimental run product. Experiments performed at room pressure generally quenched to a single blob, whereas vacuum experiments occasionally dispersed into several smaller metallic blobs upon quenching. All experimental phases, including different blobs from the same experiment, were found to the un-zoned, suggesting that the evaporation of these elements from metallic melts is not diffusion-limited (Fig. 2).
Figure 1: Examples of experimental run products
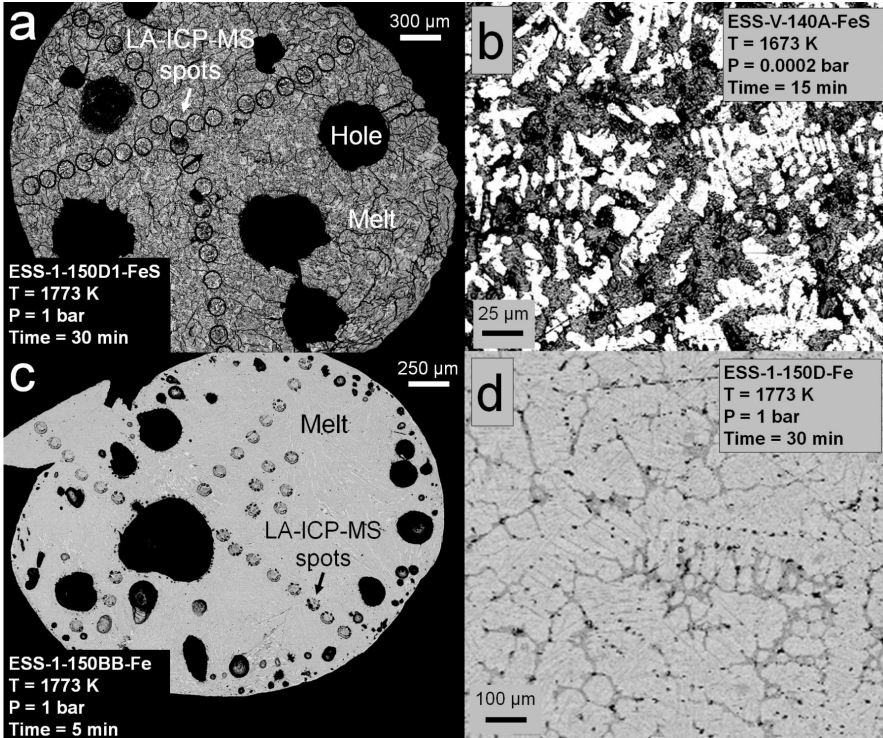
Figure 2: Concentration profiles along rim-to-rim and/or vertical LA-ICP-MS spot transects in experimental samples
The volatility or evaporative loss factor of element i (fi) was defined using the following equation: concentration of element i in experimental sample (ppm) / concentration of element i in reference material (ppm) [4]. These factors were then normalized to Ni, a non-volatile element within the explored P-T conditions, by considering f (i/Ni), defined as f i / f Ni. Figure 3 shows an example of these values for Pb in FeS liquid.
Figure 3: Experimentally determined f (Pb/Ni) values for FeS liquid
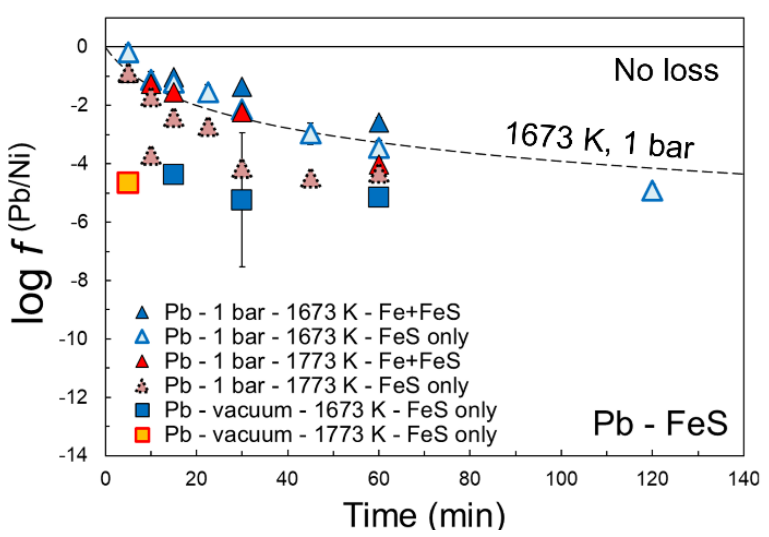
The new experimental data confirmed previous hypothesis related to the importance of S in establishing elemental volatilities (e.g., ref. 4). Some samples were so rapidly degassed that only minimum evaporative loss factors could be calculated (e.g., for Cd). Using the new experimental data, parameterizations were obtained that can be used to predict the volatility of the elements of interest [4,5] in FeS or S-free alloys as a function of T and time at a vacuum of 10-4 bar and/or 1 bar. These parameterizations were applied to predict the evaporative volatile loss during different planetary differentiation scenarios. A comparison of the new results with traditionally applied volatility models based on condensation temperatures confirms that the latter models are only partly applicable to constrain evaporative loss and particularly such loss from metallic melts [4]. The new data and parameterizations will be discussed at the meeting in light of current models that describe volatile element depletions in magmatic iron meteorite parent bodies.
References
[1] Goldstein et al. (2009) Chemie Der Erde – Geochem [2] Scott & Wasson (1975) Rev Geophys [3] Kleine et al. (2018) LPSC #2083 [4] Steenstra et al. (2023) EPSL [5] Steenstra et al., under review.
How to cite: Steenstra, E. S., Renggli, C. J., Berndt, J., and Klemme, S.: The evaporation of volatile elements from metal melts: implications for volatile element depletions in metal-rich planetesimals, Europlanet Science Congress 2024, Berlin, Germany, 8–13 Sep 2024, EPSC2024-185, https://doi.org/10.5194/epsc2024-185, 2024.