Co-evolution of organic and mineral phases during hydrothermal alteration of a simulated carbonaceous asteroid.
- 1Aix-Marseille Université, Institut Origines, UMR CNRS 7345, PIIM, Marseille 13013, France, coline.serra@univ-amu.fr
- 2Aix-Marseille Université, UMR CNRS 7325, CINAM, Marseille 13013, France
Introduction: Asteroids are primitive bodies formed from the first solids and organic matter (OM) present in the protoplanetary disc. The study of a particular class of meteorites, the carbonaceous chondrites (CC), derived from small, undifferentiated asteroids considered primitive, has demonstrated that some of them have undergone episodes of aqueous alteration. The mineralogical assemblage of the chondrite provides evidence of aqueous alteration. The formation of secondary minerals, such as phyllosilicates, can be observed. These CC can contain up to 4wt% of OM, some of which is in the form of diffuse OM intercalated in the phyllosilicates 1,2. There are many questions surrounding the history of this OM: to what extent has the OM evolved during this alteration within these mineral assemblages? Does this alteration explain the molecular diversity found in these objects today? Did the presence of OM influence the paragenesis obtained at the end of the alteration process? The co-alteration of OM and minerals under hydrothermal conditions represents a complex set of interactions that has received only limited attention in previous research. This study aimed to investigate these questions by experimentally simulating alteration conditions in the laboratory using model organic and mineral materials.
Methods: Experiments have been designed to investigate the chemical and mineralogical evolution of a hypothetical initial chondritic material exposed to hydrothermal conditions simulating asteroidal evolution. The objective of these experiments is to investigate the interactions between OM and minerals, taking into account the initial alteration conditions that integrate OM and primary minerals that may have been accreted within asteroids.
The organic material used in these experiments is hexamethylenetetramine (HMT), chosen as a model for interstellar organic compounds. This molecule (C6H12N4) constitutes about 40wt% of the organic residues recovered in interstellar ice experiments 3. The selected silicates belong to the olivine and feldspar families, as they are considered primary minerals in asteroids and are observed in the least altered chondrites 4. To further increase the mineral complexity of our study, troilite (FeS), an iron sulfide commonly observed in weakly altered meteorites, was also introduced as a model iron mineral 4,5. The experiments consist of different compositions of HMT with minerals for different durations ( 1 to 100 days) under anoxic conditions at 80°C. We experimented the co-evolution of each mineral alone with the HMT and of a mixture of the three minerals in a ratio of 2:1:2 (peridot:troilite:feldspar) with the HMT.
The mineral fraction was analysed by elemental analysis, infrared spectroscopy, electron microscopy and X-ray diffraction analyses. The diversity of organic compounds formed from HMT was characterised by gas chromatography coupled to mass spectrometry (GC-MS) and infrared spectroscopy.
Results and Discussion: For an initial HMT concentration of 30 mM, we observed different decrease over time depending on the presence and nature of minerals. The degradation of HMT was the faster in the presence of troilite with almost 50% of HMT degraded after 1 day. On the contrary, in the presence of feldspar, the lowest HMT consumption was observed even after 100 days. For peridot and the mixture of minerals, the degradation is comparable to that observed in the absence of minerals, although it occurs more rapidly over extended periods (100 days).
We have characterized by GC-MS, a wide range of chemical families produced by the degradation of HMT under hydrothermal conditions. These include carboxylic acids, diols, ester derivatives, cyanides, amides and N-bearing aromatics. We chose to focus on the amides because they dominated in all experiments, especially in the first 10 days and their formation mechanism from HMT degradation can be easily explained. The presence and nature of the minerals have a crucial influence on the formation of amides. While in some cases, the mineral tends to slow down their formation (HMT alone compared to HMT with peridot, feldspar and complex mixture), the presence of troilite clearly favours their synthesis, but only for formamide derivatives. At longer time (100 days), amide abundance is highest with peridot (Figure 1).
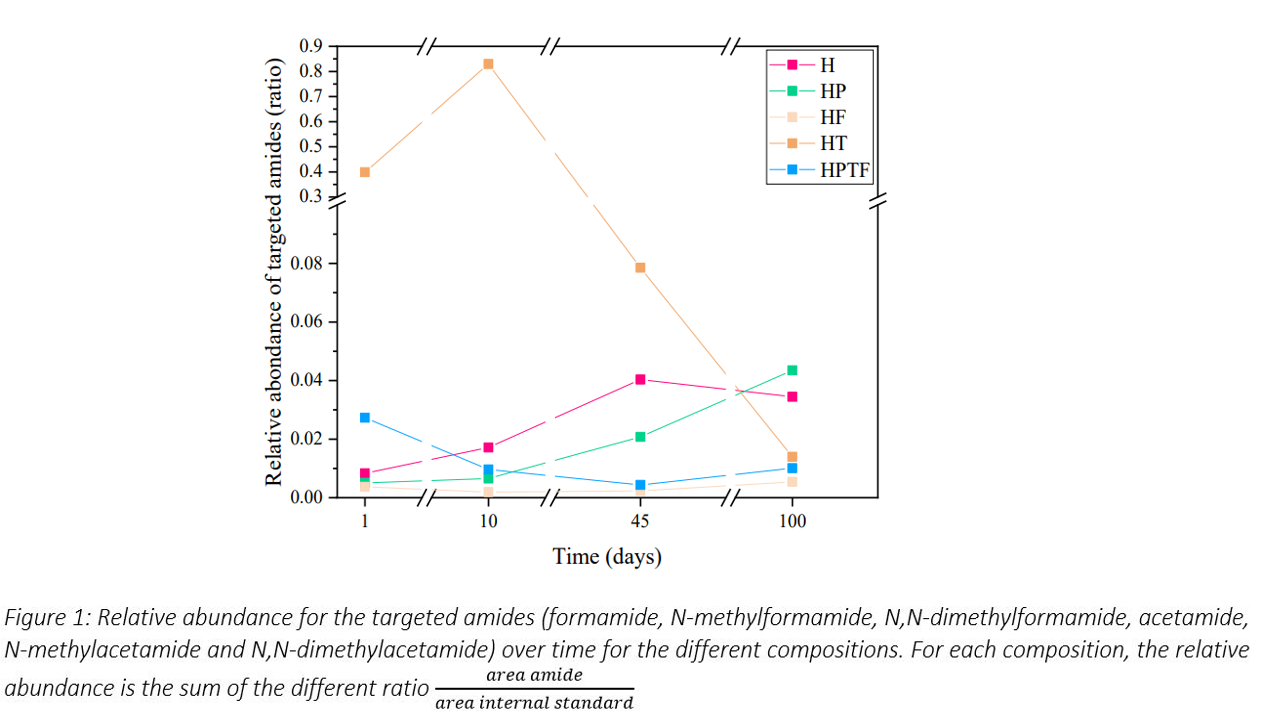
The in-situ transformations of minerals also influence the pH conditions and the release of metallic ions. We were able to identify the presence of many organic salts and chelates, which probably altered the reactivity of the system. The primary mineral has indeed undergone partial transformation into secondary phases, including amorphous silicate, phyllosilicates, or oxides (for troilite samples). Under our alteration conditions, primary minerals were transformed into secondary minerals similar to those observed in CCs 4. Nevertheless, the presence of OM has affected the formation and composition of phyllosilicates, especially from peridot. We observed differences in the nature of the phyllosilicates formed, particularly at short alteration times (<10 days). In addition, the formation of secondary phases such as phyllosilicates, can also result in the adsorption of OM, thereby rendering it less available for future reactivity.
Overall, the evolution of the organo-mineral system cannot be reduced to the sum of the two systems. In fact, the two materials interact and evolve altogether during the alteration process. Moreover, the greater the initial diversity of the organo-mineral system (comprising multiple minerals and OM), the more complex it becomes, and novel and unexpected results are obtained in comparison to the binary system. The results of our experiment simulations clearly demonstrated the significant impact that both phases can have on each other. It was observed that a wide range of molecular diversity is formed under aqueous conditions, which depends on the presence and nature of the minerals. Concurrently, the OM influences the formation of secondary mineral phases.
Aknowledgements: We acknowledge support from the Agence Nationale de la Recherche (ANR 22-CE49-0007 284 ORGAMISS – PI V. Vinogradoff). We thank Daniel Ferry with IR measurements at CINAM and Gregory Excoffier (Spectropole of the Fédération des Sciences Chimiques Marseille, Aix Marseille University) for his help with elemental analysis.
References:
(1) Le Guillou et al., Geochimica et Cosmochimica Acta 2014, 131, 368–392. https://doi.org/10.1016/j.gca.2013.11.020.
(2) Le Guillou et al., Geochimica et Cosmochimica Acta 2014, 131, 344–367. https://doi.org/10.1016/j.gca.2013.10.024.
(3) Caro et al., A&A 2003, 412 (1), 121–132. https://doi.org/10.1051/0004-6361:20031408.
(4) Brearley, Meteorites and the early solar system II 2006, 943, 587–624.
(5) Herndon et al., Nature 1975, 253 (5492), 516–518. https://doi.org/10.1038/253516a0.
How to cite: Serra, C., Vinogradoff, V., Grauby, O., Danger, G., and Duvernay, F.: Co-evolution of organic and mineral phases during hydrothermal alteration of a simulated carbonaceous asteroid., Europlanet Science Congress 2024, Berlin, Germany, 8–13 Sep 2024, EPSC2024-244, https://doi.org/10.5194/epsc2024-244, 2024.