Simulating with a Simulant: Recreating Hydrothermal Metamorphism on Enceladus
- 1Diamond Light Source, Physical Sciences - Crystallography, Didcot, United Kingdom (laura.jenkins@diamond.ac.uk)
- 2Diamond Light Source, Physical Sciences - Crystallography, Didcot, United Kingdom
Introduction
Enceladus, an icy moon of Saturn, has a subsurface ocean with large geysers that originate from hydrothermal vents. A solvent, like liquid water, and a source of energy, such as a hydrothermal vent, are both key elements in creating an environment habitable to life and are involved in theories of its origin [1]. Modelling of the interior of Enceladus suggests its silicate core is composed of undifferentiated material like that found on carbonaceous asteroids and comets [2], which are known to contain amino acids, the building blocks of life [3,4]. Understanding how Enceladus’s sea floor alters will increase the knowledge of the geology surrounding its hydrothermal vents.
Sampling of material from Enceladus’s geysers by the Cassini mission suggests hydrothermal metamorphism is occurring at temperatures of at least 150°C [2]. To better understand hydrothermal processing on Enceladus, we have heated a carbonaceous asteroidal simulant in water at 150°C to study the alteration products that formed.
Methods
CM-E carbonaceous chondrite simulant intended to be representative of the ‘Mighei-like’ carbonaceous (CM) chondrite Murchison was acquired from Space Resource Technologies, with 1.4 cm3 (1.45 g) placed in a 45 ml acid digestion vessel with 22 ml of distilled water and sealed in an inert N2 atmosphere. The vessel was then heated at 150°C for 33 days. The pressure that resulted is calculated to be 6 bars. To study changes in mineralogy, high resolution synchrotron X-ray diffraction (XRD) data was collected at the I11 beamline at Diamond Light Source both for unaltered simulant and the hydrothermally processed simulant. Proportions of different minerals were determined by Rietveld refinement using Topas 4.2. To study the composition of the liquid after hydrothermal alteration, the water from the experiment was extracted with a 450 μm diameter syringe and was analysed by X-ray fluorescence (XRF) using a PANalytical Epsilon3 XL with an Ultralene filter at the Materials Characterisation Laboratory at ISIS Neutron and Muon Source.
Results and Discussion
The CM simulant is a heterogeneous mixture of components meant to recreate textures observed on carbonaceous asteroids [6] and exact proportions of minerals are bound to vary from sample to sample. However, even given this, there are distinct differences between the mineralogy of the simulant used and the CM chondrite it is modelled after (Table 1). CM chondrites are composed mostly of serpentine minerals and tochilinite [5], whereas the simulant used is mostly made out of montmorillonite (Table 1), which lacks Fe and contains more abundant Al, Ca, and Na. Additionally, minerals that are either absent from CM chondrites or occur as terrestrial alteration products, like sulphates, pyrite, hematite, and quartz were present in the simulant (Table 1).
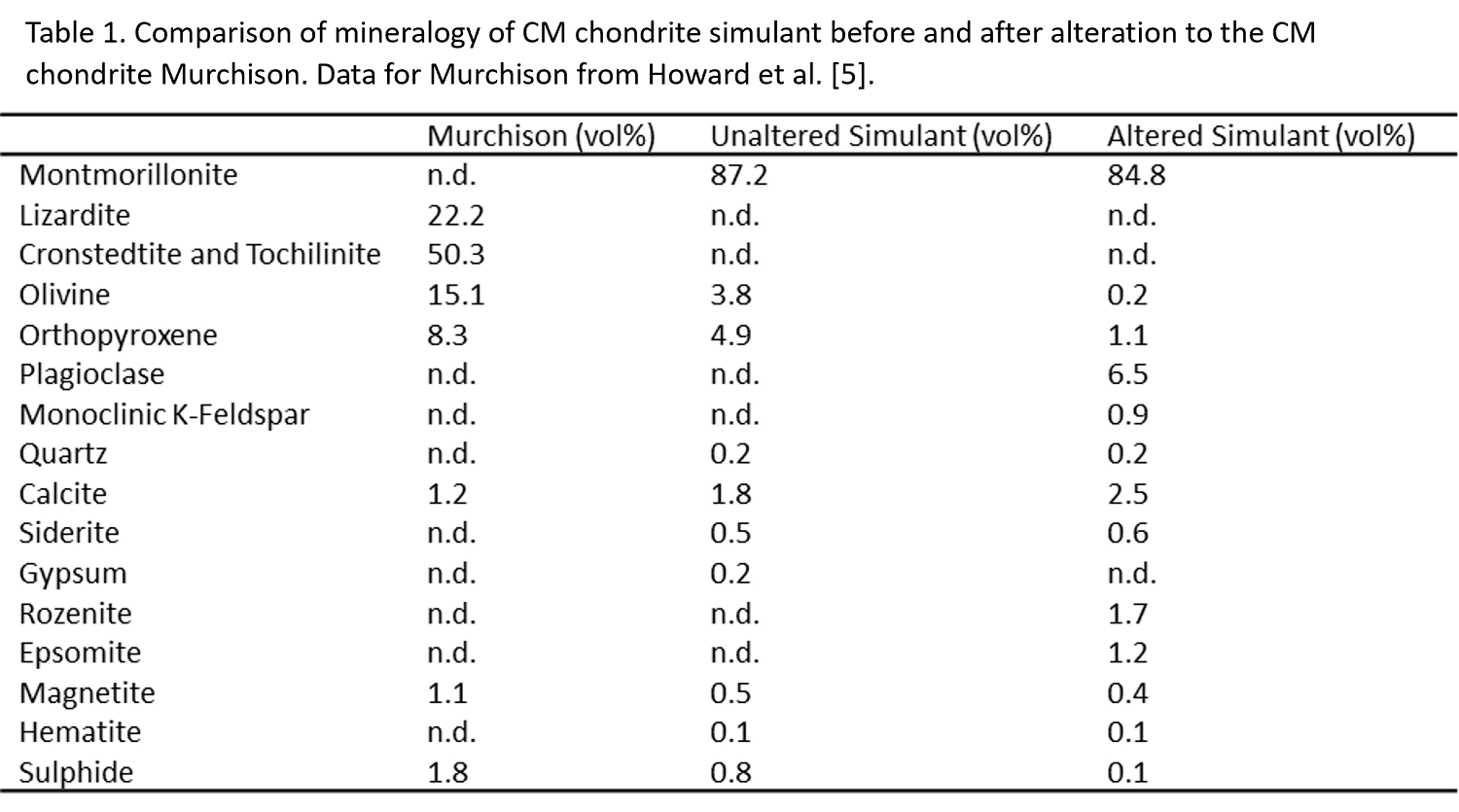
The differences between the simulant and CM chondrites defined the alteration products observed. The altered simulant contains significant amounts of feldspars and sulphates. The feldspars are likely the result of olivine and pyroxene decomposing and reacting with the montmorillonite. The K-feldspar produced is likely adularia, a low temperature hydrothermal mineral [7], while the plagioclase likely resulted as albitization (replacement of feldspar by albite) of adularia, which also occurs under low temperature hydrothermal conditions [8]. Rozenite and epsomite are sulphates in the altered simulant which were likely produced by reactions with gypsum and pyrite and other minerals (e.g., olivine) within the simulant. If CM chondrite material were used, it is likely serpentine and sulphide minerals (e.g., pyrrhotite) would be produced instead, but the breakdown of olivine and pyroxene still would have occurred as observed here.
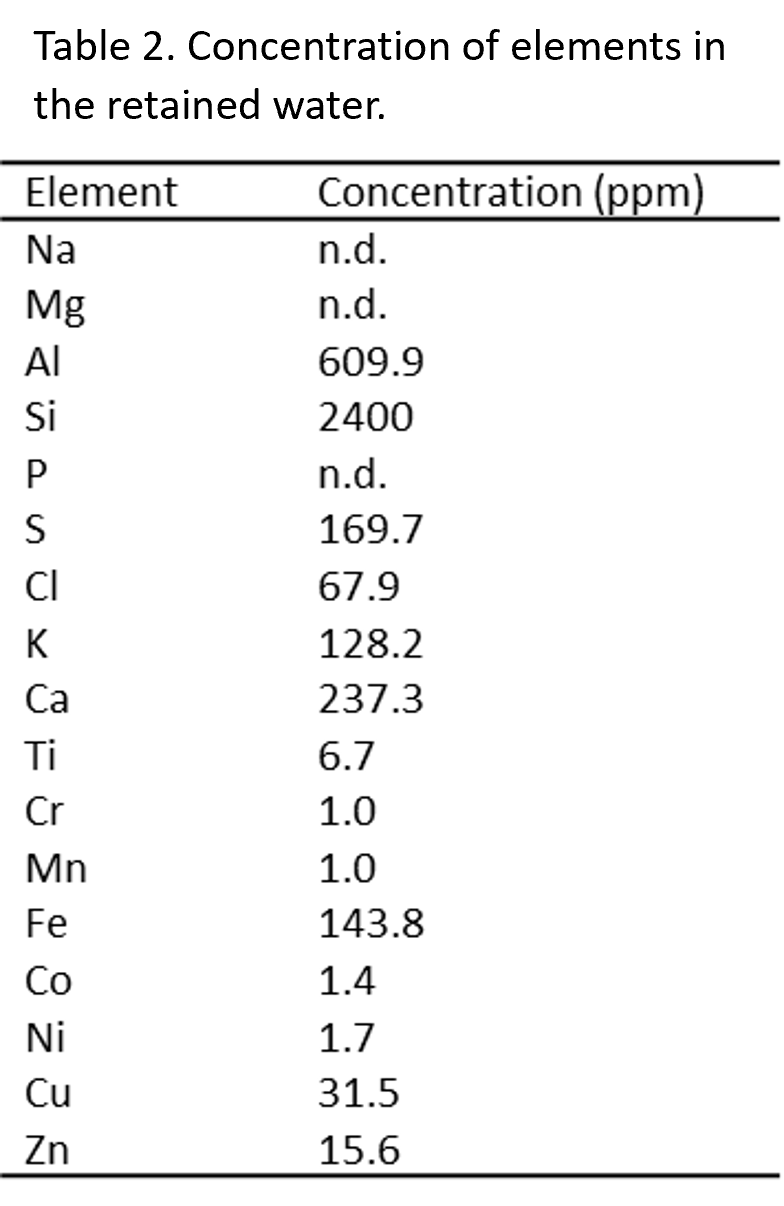
The water extracted from the autoclave following the experiment was cloudy with small particulates still suspended in it. The particulates could not be separated from the water without significant water loss so were measured alongside the water. The water mixture was found to contain an abundance of Si (Table 2), which may be attributed to sub-mm silica particles. The sizes of these silica particles are unknown, however nanometre Si-rich particles are found in Enceladus’s plumes and other similar hydrothermal environments from a reaction related to clay minerals [9]. The concentration of Si observed here is consistent with that in Saturn’s E-ring, which is produced by Enceladus [9]. However, despite this similarity, no Na was found in the water mixture (Table 2), indicating that any Na present is inside the solid sample, which is dissimilar to Enceladus’s Na-rich ocean. The montmorillonite hydrothermally altered here is chemically different than the material making up Enceladus’s core, however hydrothermal alteration of a clay mineral is likely occurring on Enceladus.
Conclusions
The recreation of hydrothermal processes on Enceladus using CM chondrite simulant resulted in the breakdown of olivine and pyroxene and the production of feldspars. This reaction is likely the result of the CM chondrite simulant containing relatively high aluminium and alkali elements compared to CM chondrite meteorites. The fluid extracted contained sub-mm particulates rich in Si and lacking in Na. The CM simulant is not chemically analogous to the seafloor of Enceladus however its clay rich nature does give it some degree of similarity.
Acknowledgements
We thank Sarah Day and Lucy Saunders for assisting with sample preparation and XRD data collection. We also thank Gavin Stanning and Daniel Nye for use of their XRF and assistance with XRF data collection. We would like to thank Raul Montes for assistance in calculating the pressures produced in this experiment.
References
[1] Corliss et al. (1981) Oceanol. Acta., 4: 59-70. [2] Sekine et al. (2015) Nat. Commun., 6: 8604. [3] Botta et al. (2002) Orig. Life. Evol. Biosph., 32: 143-163. [4] Altwegg et al. (2016) Sci. Adv., 5: e1600285. [5] Howard et al. (2011) Geochim. Cosmochim. Acta., 75: 2735-2751. [6] Britt et al. (2019) MAPS, 54: 2607-2082. [7] Steiner (1970) Mineral. Mag., 37: 916-922. [8] Chowdhury & Noble (1993) Mar. Pet. Geol. 10: 394-402. [9] Hsu et al. (2015) Nature, 519: 207-210.
How to cite: Jenkins, L., Thompson, S., and Connolly, E.: Simulating with a Simulant: Recreating Hydrothermal Metamorphism on Enceladus, Europlanet Science Congress 2024, Berlin, Germany, 8–13 Sep 2024, EPSC2024-451, https://doi.org/10.5194/epsc2024-451, 2024.