- 1Memorial University, St. John's, Canada
- 2LATMOS/CNRS, Sorbonne Université, Paris, France
- 3Columbia Astrophysics Laboratory, Columbia University, New York, USA
- 4NASA GSFC, Greenbelt, USA
Introduction: The surfaces of airless planetary bodies such as the Moon and Mercury can be subjected to several different emission processes including solar wind induced sputtering, photon stimulated desorption, and micrometeorite impact vaporization (McClintock et al. 2018; Kallio et al. 2019; Wurz et al. 2022). Many of the ejected atoms leave the surface at energies lower than the escape energy of the body and thus return to the surface, hereafter referred as low energy returning atoms (LERAs) (Burger et al. 2014). A portion of these LERAs can then be reaccommodated on the surface at an energy and composition unique from the mineral bulk. However, current global exosphere models are unable to consider the effects of LERAs nor the contribution of emission from the newly formed adsorbed layers. Instead, these models assume emission only from the body’s mineral surface, thus overlooking a potentially important exospheric source.
Previous studies have noted the surface binding energy (SBE) of surface atoms as a key parameter affecting the yield and energy distribution of different emission processes. Molecular dynamics (MD) simulations, which use an interatomic potential to simulate processes on the atomistic scale, offer a way to study these interactions without requiring mineral specific user inputs. However, the computational load of MD limits the simulation size and duration. Therefore, MD can be used to derive parameters that can be used in more efficient ejection models(Morrissey et al. 2022). No study has considered the SBE of adsorbates from relevant planetary minerals.
Methodology: In this study, we use MD simulations to study the two endmembers of Na coverage onto an SiO2 surface (i.e., 0% and 100% covered). The first case represents when individual Na atoms are adsorbed onto an initially pure SiO2 surface (i.e., without any previous adsorbed atoms). For this case we considered Na onto amorphous and crystalline SiO2. The second case represents when Na atoms have formed an initial monolayer (ML) and are instead adsorbed onto Na atoms, which will be referred to as 100% coverage. For each of these surfaces we adsorb ~400 individual Na atoms (resetting the surface each time) and test their subsequent SBE.
Results: Table 1 displays the average SBE results for 0% and 100% coverage scenarios.
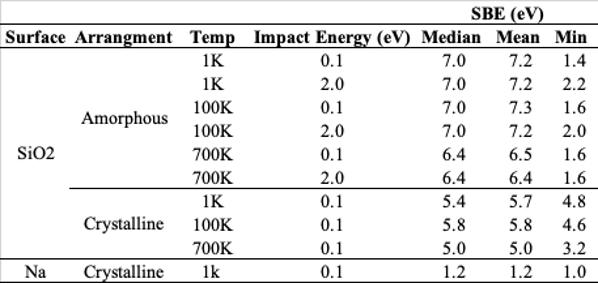
First, for the 0 % coverage case the SBEs range from ~2-12 eV with a mean value of ~6-7 eV. When the substrate is crystalline, instead of amorphous, is a smaller range of values and a decrease in the median and mean SBE by ~1.5 eV. Therefore, it is possible for adsorbed Na atoms to have significantly lower SBEs than found in crystalline albite (~8 eV), even at 0% coverage. The range of possible SBEs is also highly dependent on the crystallinity of the target, meaning weathered amorphous rims could present opportunities for more loosely bound Na.
When coverage increases to a ML there is a distinct drop in the mean SBE (~1 eV) and its range (1-2 eV). We attribute these differences to the unique bond types formed in each case. In the 0% coverage case, it is expected that the adsorbed Na atoms form ionic bonds with the free oxygen atoms on the SiO2 surface which have a high bond strength. At 100 % coverage, there are only Na-Na bonds available to be formed, which are instead metallic and have a comparatively lower bond strength.
These results agree with previous experimental work from Yakshinskiy et al. (2000) who found a distinct dependence in desorption temperature with coverage. At low coverage they see very little desorption, at moderate coverage they see peaks corresponding to high and low energy deposition, and at >1 ML coverage they see low energy desorption. Our results use atomistic modelling to demonstrate that the source of this behavior may be the different bond types being formed.
In summary, these novel results highlight the key role adsorbed Na may plan on different ejection mechanisms for Mercury. Results suggest that an initial buildup of tightly bound atoms may be necessary before binding with lower energy sites occurs. Once Na-Na bonds are formed there is a significant drop in the SBE, making ejection processes significantly more efficient. Exploring regions on Mercury where sufficient Na may accumulate to terminate surface O bonds could provide valuable insights. Further work is required to determine intermediate coverage scenarios and better understand the effect of coverage on SBE and subsequent SW-induced sputtering yield.
How to cite: Morrissey, L., Lewis, J., Ricketts, A., Leblanc, F., Savin, D., Sarantos, M., and Verkercke, S.: Adsorbed Sodium on Mercury: Atomistic Insights on the Role of Coverage , Europlanet Science Congress 2024, Berlin, Germany, 8–13 Sep 2024, EPSC2024-550, https://doi.org/10.5194/epsc2024-550, 2024.