- 1Laboratory for Astrophysics and Surface Physics, University of Virginia, 395 McCormick Road, Charlottesville, 22904 VA, USA
- 2Max Planck Institute for Plasma Physics, Wendelsteinstraße 1, 17491 Greifswald, Germany
Mercury’s exosphere is sourced from meteoritic and solar radiation interaction with the surface and is therefore a reflection of the soil composition. Curiously, sulfur has not been detected in the exosphere unlike magnesium and calcium [1]. This is despite a strong correlation between sulfur and calcium across the surface [2,3], and with magnesium in the Intercrater Plains and Heavily Cratered Terrain as detected by the X-ray spectrometer (XRS) onboard MESSENGER [4].
Defect-mediated diffusion of sulfur in troilite (FeS) and pentlandite [(Fe,Ni)9S8] has been proposed to explain observational and experimental results where a sulfur-depleted layer is capped by a Fe surface layer [5-7]. Unlike the Moon, where FeS grains are known to occur in the regolith [8], Mercury is relatively poor in iron. If the diffusion parameter found for FeS [6] were to be applied to other sulfides, the amount of available sulfur would be reduced by about an order of magnitude [9]. The presence of a metal layer formed by space weathering processes could explain why sulfur was detected by spectrometers observing the surface onboard MESSENGER [2,3,4] but not ejected at rates necessary for exospheric. In Jäggi et al. 2024 it was assumed that all sulfides express a similar sulfur diffusion due to a lack of experimental data. Therefore, in this study, we have used X-ray Photoelectron Spectroscopy to measure surface sulfur concentrations for oldhamite (CaS) and niningerite (MgS) as a function of incident ion fluence.
Sulfide pellets were pressed from powder samples (≤10 µm) in a nitrogen-purged glove tent and transferred to the XPS instrument under low vacuum to prevent atmospheric contamination. These experiments were then simulated using the Binary Collision Approximation (BCA) Monte Carlo code SDTrimSP. In Figure 1 we show the simulated evolution of the surface composition of a CaS pressed-powder pellet, irradiated with 1 keV H+, along with the XPS data. Atmospheric contamination due to oxygen and adventitious carbon leads to an initial surface that is non-stoichiometric. The remnant 15 at% of surface oxygen at fluences ≥ 1019 ions/cm2 is interpreted to be a result of the rough surface of the pressed powder sample shadowing oxidized inter-granular spaces.
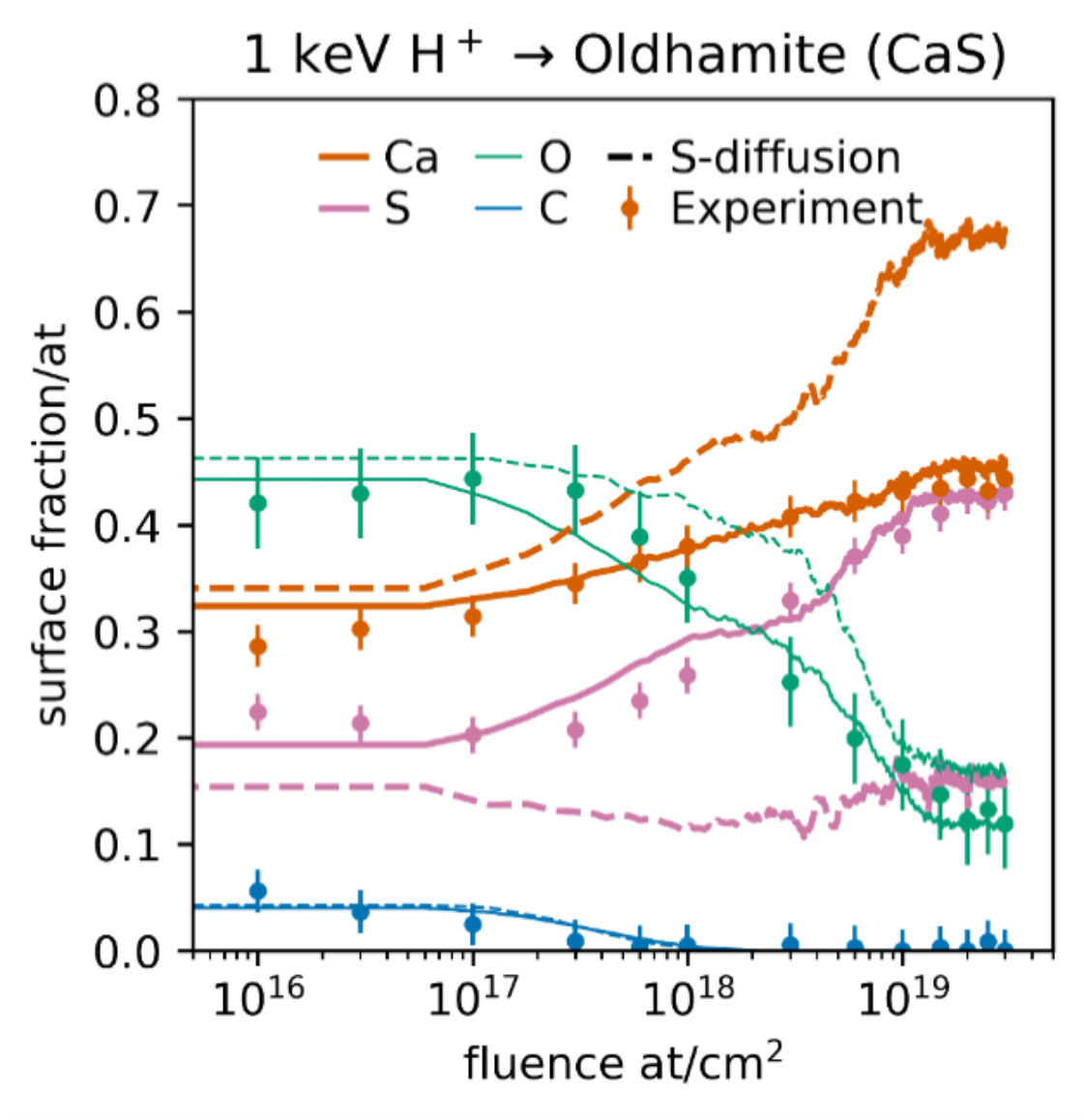
Figure 1: Irradiation of oldhamite (CaS) powder pellet with ~3 x 1013 H+/s at 1 keV. XPS data (dots) and its error, the BCA results without (line) and with diffusion (dashed line; diffusion rate from Ref. 6).
Interestingly, our measurements indicate that defect-mediated sulfur diffusion does not occur in CaS as it does in FeS; instead, a near stoichiometric Ca:S ratio is seen at the surface, after removal of adventitious carbon and the oxidized surface. We will present data for MgS and CaS, separately irradiated by H+ and He+, and make informed predictions on the behavior of Mercury’s sulfides based on our observations and diffusion simulations. In addition to compositional data, we will present changes in morphology (e.g., Fig. 2) and mid infrared reflectivity. For now, we can conclude that the surface depletion of sulfur observed when irradiating iron sulfides is not occurring in CaS. Consequently, a metal surface-layer does not form on CaS and the sputtering of sulfur is not reduced. The non-detection of sulfur in Mercury’s exosphere is, if tied to low supply of sulfur, likely the result of other processes.
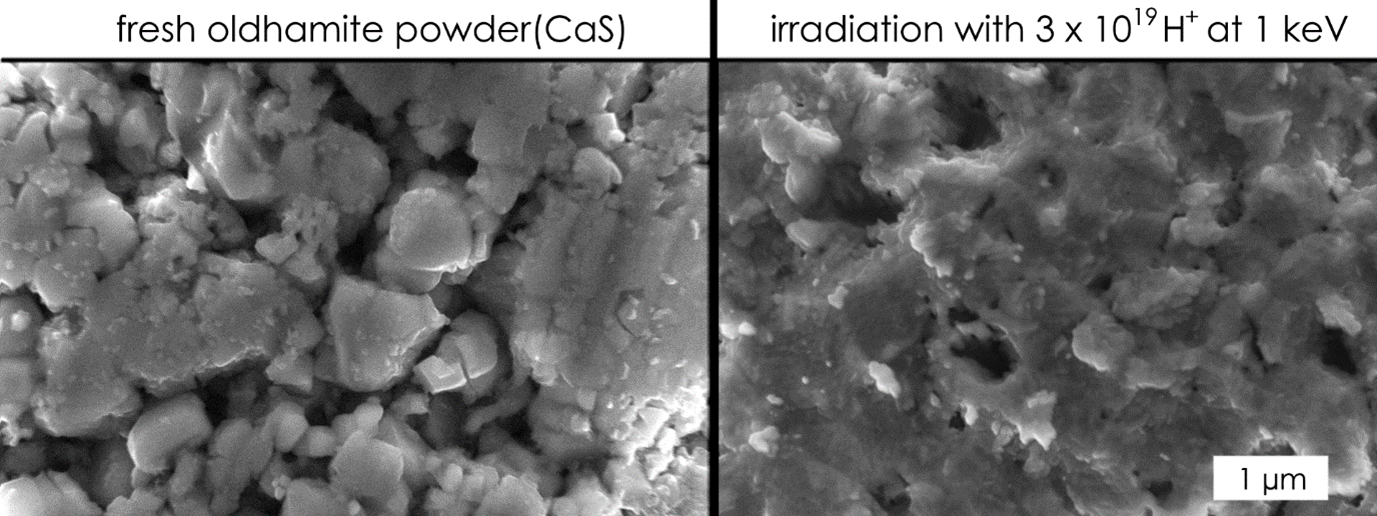
Figure 2: SEM image of pressed oldhamite powder before and after irradiation.
[1] Grava, C., et al. (2021), Space Sci. Rev. 2021 217:5, 217(5), 1–47.
[2] Nittler, L. R., et al. (2011), Science 333, 1847–1850.
[3] Weider, S. Z., et al. (2015), Earth Planet Sc. Lett. 416, 109–120.
[4] Weider, S. Z., et al. (2012), J. Geophys. Res. Planets 117, 1–15.
[5] Keller, L. P., et al. (2013), 44th LPSC, 1719, 2404.
[6] Christoph, J. M., et al. (2022), J. Geophys. Res.-Planets 127, e2021JE006916.
[7] Chaves and Thompson (2022), Earth Planets Space, 74, 124.
[8] Matsumoto, T., et al. (2021), Geochim. Cosmochim. Ac., 299, 69–84.
[9] Jäggi, N., et al. (2024), Planet. Sci. J., 5(3), 75.
How to cite: Jäggi, N., Morel, C. M., Mutzke, A., and Dukes, C. M.: Evolution of Mercury's Sulfides in the Solar Wind, Europlanet Science Congress 2024, Berlin, Germany, 8–13 Sep 2024, EPSC2024-610, https://doi.org/10.5194/epsc2024-610, 2024.