Early Stages of Diamond Rain formation in Icy Giants
- 1Theoretical Chemistry Laboratory, Sorbonne University, Paris, France
- 2International Centre for Theoretical Physics, Trieste, Italy
Little is known about the chemical transformations occurring within the interiors of Uranus and Neptune, the two icy giants of our solar system. Indeed, the extreme pressure and temperature conditions, reaching millions of atmospheres and thousands of kelvins respectively, make spacecraft exploration impossible. It is believed that between the atmosphere and the rocky core of these planets lies a dense fluid predominantly composed of a planetary mixture of water, methane, and ammonia, with pressures ranging from 1 to 1000 GPa and temperatures from 1000 to 8000 K. Understanding the transformations underlying the chemistry of the planetary mixture in extreme conditions could provide valuable insights for rationalizing some of the physical properties of these planets. In this regard, an intriguing hypothesis, known as the "diamonds in the sky hypothesis," suggests that the formation of diamonds and superionic water from the planetary mixture could provide a rationalization for the unusual magnetic field and luminosity of icy giants.
This hypothesis has been supported by numerous experimental works based on Laser Heated Diamond Anvil Cells (LHDAC) techniques, which have provided evidences of nanodiamond formation from various C/H/O mixtures in extreme conditions. Additionally, theoretical studies based on ab initio molecular dynamics have provided insights into the physical properties of the planetary mixtures, such as the lifetime of chemical bonds, bandgap, and Hugoniot curves. However, the limited accessible timescale in the case of ab initio MD and the challenges in characterizing the process "in situ" from experiments have hindered the exploration of the mechanisms and free energies underlying the transition from C/H/O/N and C/H/O planetary mixtures to nanodiamond.
In the poster, I will present the results of our computational study on characterizing the reaction pathways starting from water/methane mixtures and leading to diamond clusters, from both a mechanistic and thermokinetic perspective. To achieve this, we developed a computational protocol combining ab initio MD, enhanced sampling techniques (metadynamics, umbrella sampling), and shooting techniques (committor analysis). The enhanced sampling techniques allowed us to explore the reactivity of the planetary mixture, overcoming current limitations in time-scale and directly considering the effects of pressure and temperature on the computed mechanism and associated free energies. Analysis of the shooting trajectories, along with electronic topological descriptor analysis (ELF and Wannier analysis), refined the transition pathways and characterized the structure and electronic properties of the transition states. Numerous simulations were conducted to cover the P-T thermodynamics diagram of the planetary mixture in the 5-90 GPa and 1500-3500 K ranges. Observation of unbiased MD trajectories and free energy estimations revealed that diamond cluster formation from CH4/H2O is possible above 3000 K and 30 GPa. Under these conditions, as carbon growth progresses, the formation of alcohols and hydrocarbons of increasing complexity is observed through reactions of chain elongation, branching, and cyclization. These reactions are predominantly dictated by a carbocation chemistry, involving the formation of transient (highly reactive) carbocation intermediates and the production of molecular hydrogen. Our simulations reveal that water plays a key role in this process. Specifically, the formation of reactive carbocation intermediates, directly responsible for the observed carbon growth, is facilitated by a proton transfer from a hydronium ion (H3O+) to the carbon of a hydrocarbon or alcohol species. We find that the ease of such exotic C-O proton transfer is directly related to the degree of water dissociation in such extreme conditions.
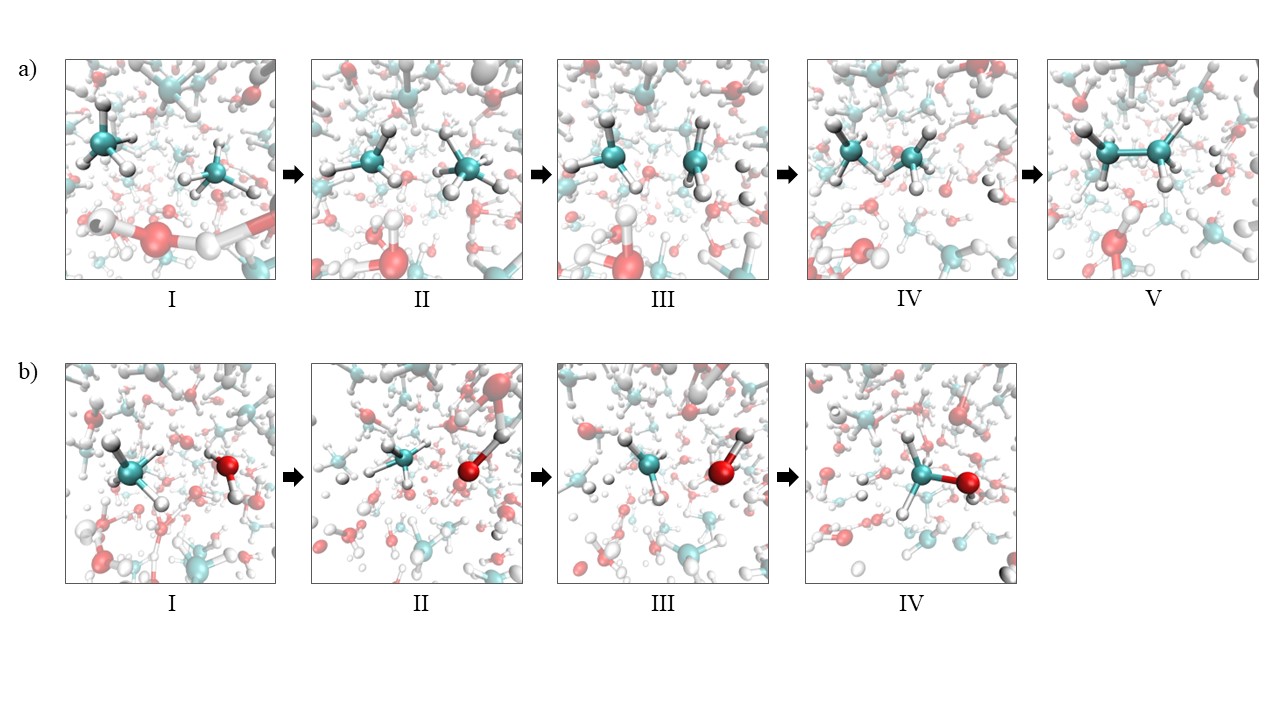
Fig1. Panel a : Snapshots of reactive structures along the observed formation of ethane. The methyl cation CH3+ (structure III), formed by the dissociation of the intermediate CH5+ ion (structure II) with release of an H2 molecule, reacts with a methane molecule (structure IV) to formed ethane with release of an H+ ion (structure V). Panel b : Snapshots of reactive structures along the observed formation of methanol. The methyl cation CH3+ (structure III), formed directly with release of an H2 molecule, reacts with a water molecule to formed methanol with release of an H+ ion (structure IV).
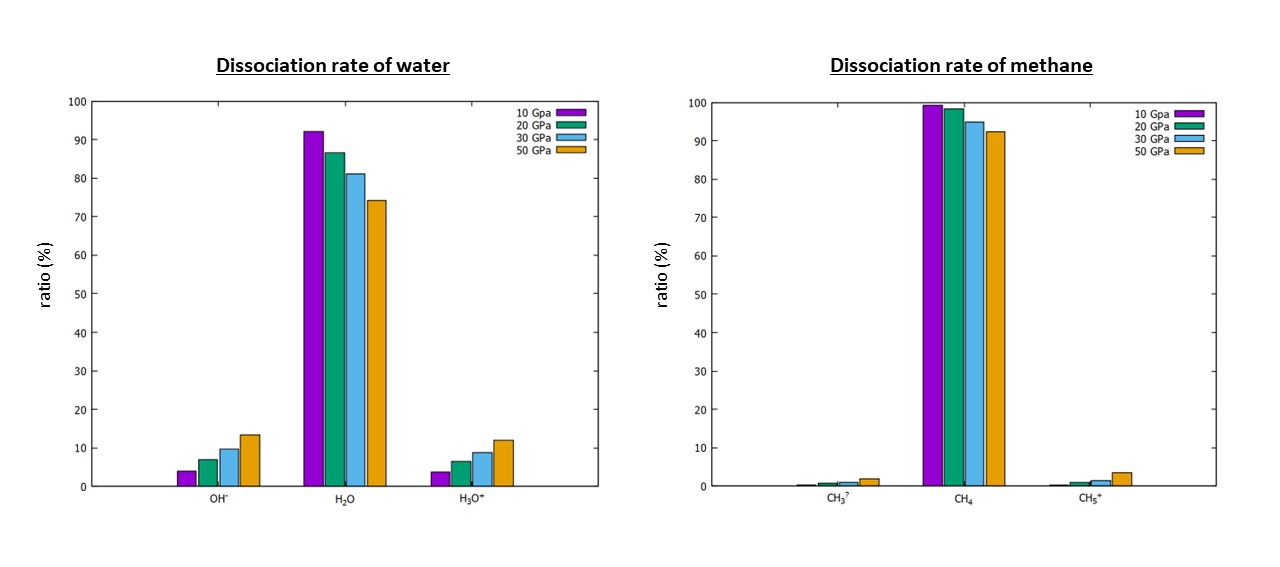
Fig2. Effect of pressure on the dissociation rate of both water and methane from 10 GPa to 50 GPa at 3000 K.
How to cite: Thévenet, T., Scandolo, S., Markovits, A., and Siro Brigiano, F.: Early Stages of Diamond Rain formation in Icy Giants, Europlanet Science Congress 2024, Berlin, Germany, 8–13 Sep 2024, EPSC2024-990, https://doi.org/10.5194/epsc2024-990, 2024.