- 1Open University, AstrobiologyOU, United Kingdom of Great Britain – England, Scotland, Wales (mark.fox-powell@open.ac.uk)
- 22Department of Chemistry, University College London, 20 Gordon Street, London WC1H 0AJ, UK
- 33School of Physical Sciences, Open University, Walton Hall, Milton Keynes MK7 6AA
- 44ISIS Neutron and Muon Source, Rutherford Appleton Laboratory, Didcot OX11 0QX, UK
- 55Diamond Light Source, Harwell Campus, Didcot, OX11 0DE, UK
Background
Sodium chloride (NaCl), the most common salt on Earth, has been detected at several icy worlds that could be habitable in the present day, including Europa [1], Enceladus [2], Ganymede [3] and Ceres [4], providing evidence that salty liquid water from their interiors has been delivered to their surfaces. Areas that have experienced the emplacement of subsurface fluids through mechanisms such as plumes could contain a record of recently exposed ocean material and thus provide information on ocean chemistry and potential habitability. Identifying such regions will be a major priority for upcoming missions such as ESA’s JUpiter ICy moons Explorer (JUICE) and NASA’s Europa Clipper.
Here, we report the discovery of a metastable NaCl dihydrate formed through rapid freezing of a NaCl solution at ambient pressure (Fig. 1) [5]. This new NaCl hydrate expands on the recently identified NaCl hydrates formed in high-pressure experiments [6], and together with these reveals a rich phase behaviour in the low temperature Na-Cl-H2O system that had been overlooked for over 200 years. Using synchrotron X-ray and neutron powder diffraction, we show that the metastable form transforms irreversibly to the stable hydrate hydrohalite above 190 K, exothermically releasing 3.47 kJ mol-1 of latent heat. Additionally, we used Raman and near-infrared (NIR) reflectance spectroscopy to show experimentally that the solid phase composition of NaCl-bearing ices varies as a function of fluid cooling rate, promising a means of reconstructing the formation history of NaCl-bearing icy world surface materials from remote measurements of their composition.
Methods
NaCl solutions were frozen from room temperature to liquid nitrogen (LN2) temperature (~77 K) using multiple techniques that allowed us to probe a wide range of cooling rates spanning < 1 K min-1 to > 107 K min-1. Neutron and X-ray diffraction (XRD) were carried out at ISIS Neutron and Muon Source and Diamond Light Source, UK, respectively. We exploited the diagnostic Raman signatures of the stable and metastable hydrates [5] to identify the cooling rates required for their formation, and how these varied with NaCl concentration. NIR spectra were recorded at wavelengths between 1.0 and 2.5 micron from powdered samples at 77 K.
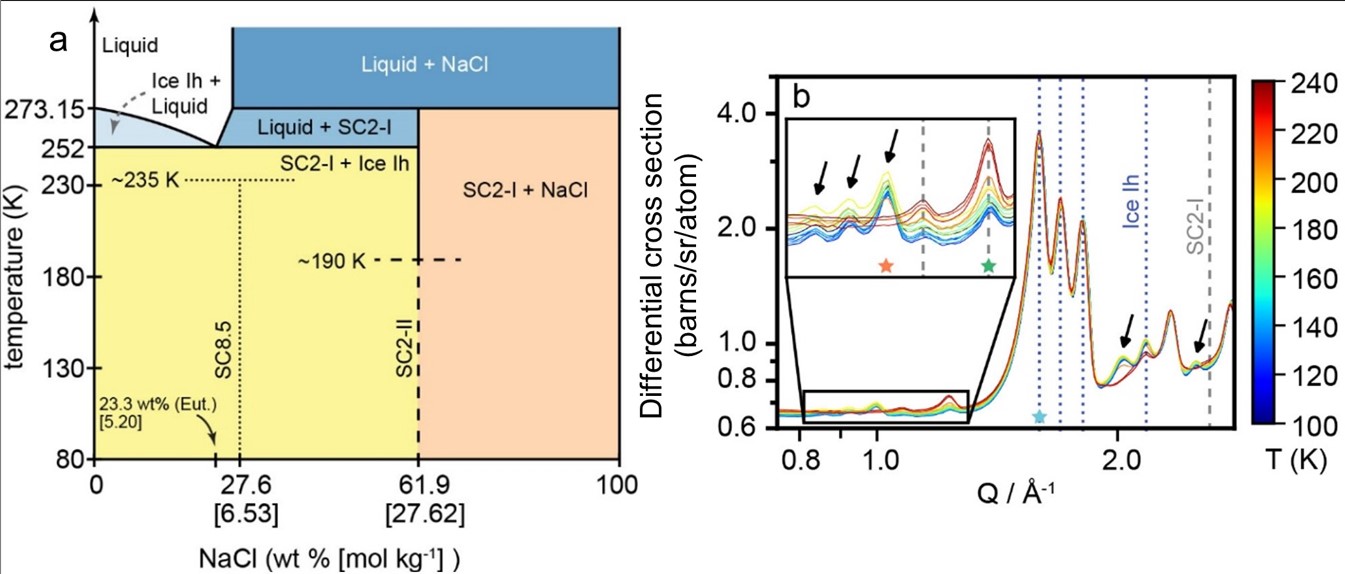
Figure 1. (a) Updated phase diagram of the low-temperature NaCl-H2O system incorporating phase behavior of high-pressure hydrate 2NaCl·17H2O (SC8.5 [6]) and proposed metastable dihydrate (SC2-II, this study, [5]). Dashed lines indicate observed metastable transition temperatures to hydrohalite (SC2-I) and ice Ih. (b) Neutron diffraction patterns for a flash frozen sample that has been heated. Bragg peaks for the new NaCl hydrate are marked with arrows, while Bragg peaks for hydrohalite (SC2-I) and ice Ih are marked with dashed lines (reproduced from [5]).
Results and Discussion
Our findings contribute to a new recognition of overlooked structural diversity and phase behavior complexity in the low-temperature NaCl-H2O system. We found that the newly discovered hydrate is a dihydrate structurally related to hydrohalite, with a proposed crystal structure comprising a 3 × 1 × 3 supercell of the hydrohalite unit cell. Because the metastable hydrate forms from liquid solutions at low pressures, it could feasibly form directly through cooling of ocean water at the surface or within the shallow ice shells of icy worlds and remain stable unless warmed above ~190 K. At active icy worlds such as Enceladus and Europa, rapid cooling of fluids could be achieved in various geological scenarios including plumes and chaos formation.
We found that the phase composition of NaCl-bearing ice is dependent on the cooling rate, indicating that compositional properties of NaCl-rich ices can act as a record of thermal history. At rates below ~90 K min-1, only the stable hydrohalite was produced. The metastable phase formed in distinct cooling rate regimes either in combination with hydrohalite, or as the sole NaCl phase. In addition, we used XRD to confirm that vitreous glass forms at the fastest rates, a phenomenon which has been proposed by previous studies [7,8]. Finally, we show that the new hydrate possesses near-infrared spectral features that are distinct from hydrohalite and thus could be used to identify it with existing or future remote sensing observations.
Our data show that in such regions distinguishing between different NaCl phase assemblages holds great promise in reconstructing the formational cooling rate of salty surface materials. Connecting surface composition to ice shell processes is a next major frontier in understanding the geology of icy worlds, a challenge that can be addressed by combining laboratory insights with new observations from upcoming planetary missions including NASA’s Europa Clipper and ESA’s JUICE.
References
[1] S.K. Trumbo et al. (2019). Sci. Adv., 5, eaaw7123
[2] F. Postberg et al., (2009). Nature, 459, 1098–1101
[3] F. Tosi et al., (2024). Nat. Astron., 8, 82–93
[4] M.C. De Sanctis et al., (2020) Nat. Astron. 4, 786–793
[5] R.E. Hamp et al. (2024). J. Phys. Chem. Lett. 15(50), 12301–12308
[6] Journaux et al., (2023). PNAS, 120 (9) e2217125120
[7] M.G. Fox-Powell & C.R. Cousins, (2021). J. Geophys. Res. Planets, 126, e2020JE006628
[8] F. Klenner et al. (2025) Planet. Sci. J. 6 (65)
How to cite: Hamp, R., Salzmann, C., Fawdon, P., Amato, Z., Beaumont, M., Chinnery, H., Henry, P., Headen, T., Perera, L., Thompson, S., and Fox-Powell, M.: Metastable hydrate of sodium chloride: A new mineralogical indicator of rapid freezing of brines at icy worlds, EPSC-DPS Joint Meeting 2025, Helsinki, Finland, 7–12 Sep 2025, EPSC-DPS2025-1413, https://doi.org/10.5194/epsc-dps2025-1413, 2025.